Tranexamic Acid Prescribing Information
Tranexamic acid injection is indicated in patients with hemophilia for short-term use (2 to 8 days) to reduce or prevent hemorrhage and reduce the need for replacement therapy during and following tooth extraction.
Injection: 1,000 mg/10 mL (100 mg/mL) clear and colorless solution in single-dose ampules
Injection: 1,000 mg/10 mL (100 mg/mL) clear and colorless solution in single-dose vials
Tranexamic acid injection is contraindicated:
- As a neuraxial (i.e., intrathecal, epidural) injection [see Warnings and Precautions (5.1)].
- In patients with subarachnoid hemorrhage. Anecdotal experience indicates that cerebral edema and cerebral infarction may be caused by tranexamic acid injection in such patients.
- In patients with active intravascular clotting [see Warnings and Precautions (5.2)].
- In patients with hypersensitivity to tranexamic acid or any of the ingredients [see Warnings and Precautions (5.4)].
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Risk of Medication Errors Due to Incorrect Route of Administration [see Warnings and Precautions (5.1)]
- Thromboembolic Risk [see Warnings and Precautions (5.2)]
- Seizures [see Warnings and Precautions (5.3)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.4)]
- Visual Disturbances [see Warnings and Precautions (5.5)]
- Dizziness [see Warnings and Precautions (5.6)]
Tranexamic acid is trans-4-(aminomethyl)cyclohexanecarboxylic acid, an antifibrinolytic agent. Tranexamic acid is a white crystalline powder. The structural formula is
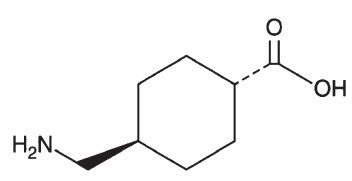
Each mL of the sterile solution for intravenous injection contains 100 mg tranexamic acid and Water for Injection to 1 mL. The aqueous
solution for injection has a pH of 6.5 to 8.0.