Trexall Prescribing Information
- Methotrexate can cause embryo-fetal toxicity, including fetal death.For non-neoplastic diseases, methotrexate is contraindicated inpregnancy.For neoplastic diseases, advise females and males of reproductive potential to use effective contraception[see.Contraindications (), Warnings and Precautions (
4 CONTRAINDICATIONSTREXALL is contraindicated in:- Pregnant women receiving TREXALL for treatment of non-neoplastic diseases[see Warnings and Precautions , and Use in Specific Populations ].
- Patients with a history of a severe hypersensitivity reactions, including anaphylaxis, to TREXALL[see Warnings and Precautions ].
- In pregnancy for non-neoplastic diseases (4)
- History of severe hypersensitivity to TREXALL (4)
), Use in Specific Populations (5.1 Embryo-Fetal ToxicityBased on published reports and its mechanism of action, methotrexate can cause fetal harm, including fetal death, when administered to a pregnant woman. Methotrexate is contraindicated for use in pregnant women receiving TREXALL for the treatment of non-malignant diseases. Advise pregnant women with neoplastic diseases of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with TREXALL and for 6 months after the final dose. Advise males with female partners of reproductive potential to use effective contraception during TREXALL treatment and for 3 months after the final dose[see Contraindications , Use in Specific Populations ].,8.1 PregnancyRisk SummaryMethotrexate is contraindicated in pregnant women with non-neoplastic diseases
[see Contraindications ].Based on published reports and its mechanism of action
[see Clinical Pharmacology ], methotrexate can cause embryo-fetal toxicity and fetal death when administered to a pregnant woman. There are no animal data that meet current standards for nonclinical developmental toxicity studies. Advise pregnant women with neoplastic diseases of the potential risk to a fetus.In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
DataHuman DataPublished data from case reports, literature reviews, and observational studies report that methotrexate exposure during pregnancy is associated with an increased risk of embryo-fetal toxicity and fetal death. Methotrexate exposure during the first trimester of pregnancy is associated with an increased incidence of spontaneous abortions and multiple adverse developmental outcomes, including skull anomalies, facial dysmorphism, central nervous system abnormalities, limb abnormalities, and sometimes cardiac anomalies and intellectual impairment. Adverse outcomes associated with exposure during second and third trimesters of pregnancy include intrauterine growth restriction and functional abnormalities. Because methotrexate is widely distributed and persists in the body for a prolonged period, there is a potential risk to the fetus from preconception methotrexate exposure.
A prospective multicenter study evaluated pregnancy outcomes in women taking methotrexate less than or equal to 30 mg/week after conception. The rate of spontaneous abortion and miscarriage in pregnant women exposed to methotrexate was 42% (95% confidence interval [95% CI] 29, 59), which was higher than in unexposed patients with autoimmune disease (22%; 95% CI: 17, 30) and unexposed patients with nonautoimmune disease (17%; 95% CI: 13, 23). Of the live births, the rate of major birth defects in pregnant women exposed to methotrexate after conception was higher than in unexposed patients with autoimmune disease (adjusted odds ratio (OR) 1.8 [95% CI: 0.6, 6]) and unexposed patients with non-autoimmune disease (adjusted OR 3.1 [95% CI: 1, 10]) (2.9%). Major birth defects associated with pregnancies exposed to methotrexate after conception were not always consistent with methotrexate-associated adverse developmental outcomes.
)]8.3 Females
and Males of Reproductive PotentialMethotrexate can cause malformations and fetal death at doses less than or equal to the recommended clinical doses
[Use in Specific Populations ].Pregnancy TestingVerify the pregnancy status of females of reproductive potential prior to initiating methotrexate
[see Contraindications , Use in Specific Populations ].ContraceptionFemalesAdvise females of reproductive potential to use effective contraception during treatment with methotrexate and for 6 months after the final dose.
MalesMethotrexate can cause chromosomal damage to sperm cells. Advise males with female partners of reproductive potential to use effective contraception during treatment with methotrexate and for 3 months after the final dose.
InfertilityFemalesBased on published reports of female infertility after methotrexate, advise females of reproductive potential that methotrexate can cause impairment of fertility and menstrual dysfunction during treatment with methotrexate and after the final dose. It is not known if the infertility may be reversed in all affected females.
MalesBased on published reports of male infertility after methotrexate, advise males that methotrexate can cause oligospermia or infertility during treatment with methotrexate and after the final dose. It is not known if the infertility may be reversed in all affected males.
- Methotrexate is contraindicated in patients with a history of severe hypersensitivity reactions to methotrexate, including anaphylaxis[Contraindications (.), Warnings and Precautions (
4 CONTRAINDICATIONSTREXALL is contraindicated in:- Pregnant women receiving TREXALL for treatment of non-neoplastic diseases[see Warnings and Precautions , and Use in Specific Populations ].
- Patients with a history of a severe hypersensitivity reactions, including anaphylaxis, to TREXALL[see Warnings and Precautions ].
- In pregnancy for non-neoplastic diseases (4)
- History of severe hypersensitivity to TREXALL (4)
)]5.2 Hypersensitivity ReactionsHypersensitivity reactions, including anaphylaxis, can occur with TREXALL[see Contraindications , Adverse Reactions ].If anaphylaxis or other serious hypersensitivity reaction occurs, immediately and permanently discontinue TREXALL[see Dosage and Administration ]. - Serious adverse reactions, including death, have been reported with methotrexate. Closely monitor for adverse reactions of the bone marrow, gastrointestinal tract, liver, lungs, skin, and kidneys. Withhold or discontinue methotrexate as appropriate.[Warnings and Precautions (,,
5.3
MyelosuppressionTREXALL suppresses hematopoiesis and can cause severe and life-threatening pancytopenia, anemia, leukopenia, neutropenia, and thrombocytopenia[see Adverse Reactions ].Obtain blood counts at baseline, periodically during treatment, and as clinically indicated. Monitor patients for clinical complications of myelosuppression. Withhold, dose reduce, or discontinue methotrexate taking into account the impor
tance of TREXALL treatment in the context of the severity of the disease being treated, the severity of the adverse drug reaction, and availability of alternative therapy[see Dosage and Administration ].,5.4
Gastrointestinal ToxicityDiarrhea, vomiting, nausea, and stomatitis occurred in up to 10% of patients receiving TREXALL for treatment of non-neoplastic diseases. Hemorrhagic enteritis and fatal intestinal perforation have been reported[see Adverse Reactions ]. Patients with peptic ulcer disease or ulcerative colitis are at a greater risk of developing severe gastrointestinal adverse reactions[see Drug Interactions ].Withhold or discontinue TREXALL for severe gastrointestinal toxicity taking into account the importance of TREXALL treatment in the context of the severity of the disease being treated, the severity of the adverse drug reaction, and availability of alternative therapy[see Dosage and Administration ].5.5
HepatotoxicityTREXALL can cause severe and potentially irreversible hepatotoxicity, including fibrosis, cirrhosis, and fatal liver failure
[see Adverse Reactions ]. The safety of TREXALL in patients with hepatic disease is unknown.The risk of hepatotoxicity is increased with heavy alcohol consumption. In patients with psoriasis, fibrosis or cirrhosis may occur in the absence of symptoms or abnormal liver tests; the risk of hepatotoxicity appears to increase with total cumulative dose and generally occurs after receipt of a total cumulative dose of 1.5 g or more.
Monitor liver tests at baseline, periodically during treatment and as clinically indicated. Withhold or discontinue TREXALL taking into account the importance of TREXALL treatment in the context of the severity of the disease being treated, the severity of the adverse drug reaction, and availability of alternative therapy
[see Dosage and Administration ].Monitor patients for pulmonary toxicity and withhold or discontinue methotrexate taking into account the importance of TREXALL treatment in the context of the severity of the disease being treated, the severity of the adverse drug reaction, and availability of alternative therapy[see Dosage and Administration ].,5.7
Dermatologic ReactionsSevere, including fatal dermatologic reactions, such as toxic epidermal necrolysis, Stevens- Johnson syndrome, exfoliative dermatitis, skin necrosis, and erythema multiforme, can occur with TREXALL[see Adverse Reactions ].Exposure to ultraviolet radiation while taking methotrexate may aggravate psoriasis.TREXALL can cause radiation recall dermatitis and photodermatitis (sunburn) reactivation.Monitor patients for dermatologic toxicity and withhold or permanently discontinue TREXALL for severe dermatologic reactions taking into account the importance of TREXALL treatment in the context of the severity of the disease being treated, the severity of the adverse drug reaction, and availability of alternative therapy[see Dosage and Administration ]. Advise patients to avoid excessive sun exposure and use sun protection measures.)]5.8
Renal ToxicityTREXALL can cause renal toxicity, including irreversible acute renal failure[see Adverse Reactions ].Monitor renal function at baseline, periodically during treatment and as clinically indicated. Withhold or discontinue TREXALL for severe renal toxicity taking into account the importa
nce of TREXALL treatment in the context of the severity of the disease being treated, the severity of the adverse drug reaction, and availability of alternative therapy[see Dosage and Administration ].Administer glucarpidase in patients with toxic plasma methotrexate concentrations (> 1 micromole per liter) and delayed TREXALL clearance due to impaired renal function. Refer to the glucarpidase prescribing information for additional information.
Tablets:
5 mg: Green, oval-shaped, film-coated, scored, biconvex tablet. Debossed with stylized
7.5 mg: Blue, oval-shaped, film-coated, scored, biconvex tablet. Debossed with stylized
10 mg: Pink, oval-shaped, film-coated, scored, biconvex tablet. Debossed with stylized
15 mg: Purple, oval-shaped, film-coated, scored, biconvex tablet. Debossed with stylized
- Pregnant women receiving TREXALL for treatment of non-neoplastic diseases[see Warnings and Precautions (.), and Use in Specific Populations (
5.1 Embryo-Fetal ToxicityBased on published reports and its mechanism of action, methotrexate can cause fetal harm, including fetal death, when administered to a pregnant woman. Methotrexate is contraindicated for use in pregnant women receiving TREXALL for the treatment of non-malignant diseases. Advise pregnant women with neoplastic diseases of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with TREXALL and for 6 months after the final dose. Advise males with female partners of reproductive potential to use effective contraception during TREXALL treatment and for 3 months after the final dose[see Contraindications , Use in Specific Populations ].,8.1 PregnancyRisk SummaryMethotrexate is contraindicated in pregnant women with non-neoplastic diseases
[see Contraindications ].Based on published reports and its mechanism of action
[see Clinical Pharmacology ], methotrexate can cause embryo-fetal toxicity and fetal death when administered to a pregnant woman. There are no animal data that meet current standards for nonclinical developmental toxicity studies. Advise pregnant women with neoplastic diseases of the potential risk to a fetus.In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
DataHuman DataPublished data from case reports, literature reviews, and observational studies report that methotrexate exposure during pregnancy is associated with an increased risk of embryo-fetal toxicity and fetal death. Methotrexate exposure during the first trimester of pregnancy is associated with an increased incidence of spontaneous abortions and multiple adverse developmental outcomes, including skull anomalies, facial dysmorphism, central nervous system abnormalities, limb abnormalities, and sometimes cardiac anomalies and intellectual impairment. Adverse outcomes associated with exposure during second and third trimesters of pregnancy include intrauterine growth restriction and functional abnormalities. Because methotrexate is widely distributed and persists in the body for a prolonged period, there is a potential risk to the fetus from preconception methotrexate exposure.
A prospective multicenter study evaluated pregnancy outcomes in women taking methotrexate less than or equal to 30 mg/week after conception. The rate of spontaneous abortion and miscarriage in pregnant women exposed to methotrexate was 42% (95% confidence interval [95% CI] 29, 59), which was higher than in unexposed patients with autoimmune disease (22%; 95% CI: 17, 30) and unexposed patients with nonautoimmune disease (17%; 95% CI: 13, 23). Of the live births, the rate of major birth defects in pregnant women exposed to methotrexate after conception was higher than in unexposed patients with autoimmune disease (adjusted odds ratio (OR) 1.8 [95% CI: 0.6, 6]) and unexposed patients with non-autoimmune disease (adjusted OR 3.1 [95% CI: 1, 10]) (2.9%). Major birth defects associated with pregnancies exposed to methotrexate after conception were not always consistent with methotrexate-associated adverse developmental outcomes.
)]8.3 Females
and Males of Reproductive PotentialMethotrexate can cause malformations and fetal death at doses less than or equal to the recommended clinical doses
[Use in Specific Populations ].Pregnancy TestingVerify the pregnancy status of females of reproductive potential prior to initiating methotrexate
[see Contraindications , Use in Specific Populations ].ContraceptionFemalesAdvise females of reproductive potential to use effective contraception during treatment with methotrexate and for 6 months after the final dose.
MalesMethotrexate can cause chromosomal damage to sperm cells. Advise males with female partners of reproductive potential to use effective contraception during treatment with methotrexate and for 3 months after the final dose.
InfertilityFemalesBased on published reports of female infertility after methotrexate, advise females of reproductive potential that methotrexate can cause impairment of fertility and menstrual dysfunction during treatment with methotrexate and after the final dose. It is not known if the infertility may be reversed in all affected females.
MalesBased on published reports of male infertility after methotrexate, advise males that methotrexate can cause oligospermia or infertility during treatment with methotrexate and after the final dose. It is not known if the infertility may be reversed in all affected males.
- Patients with a history of a severe hypersensitivity reactions, including anaphylaxis, to TREXALL[see Warnings and Precautions (.)]
5.2 Hypersensitivity ReactionsHypersensitivity reactions, including anaphylaxis, can occur with TREXALL[see Contraindications , Adverse Reactions ].If anaphylaxis or other serious hypersensitivity reaction occurs, immediately and permanently discontinue TREXALL[see Dosage and Administration ].
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypersensitivity Reactions [see Warnings and Precautions ()]
5.2 Hypersensitivity ReactionsHypersensitivity reactions, including anaphylaxis, can occur with TREXALL[see Contraindications , Adverse Reactions ].If anaphylaxis or other serious hypersensitivity reaction occurs, immediately and permanently discontinue TREXALL[see Dosage and Administration ]. - Myelosuppression [see Warnings and Precautions ()]
5.3
MyelosuppressionTREXALL suppresses hematopoiesis and can cause severe and life-threatening pancytopenia, anemia, leukopenia, neutropenia, and thrombocytopenia[see Adverse Reactions ].Obtain blood counts at baseline, periodically during treatment, and as clinically indicated. Monitor patients for clinical complications of myelosuppression. Withhold, dose reduce, or discontinue methotrexate taking into account the impor
tance of TREXALL treatment in the context of the severity of the disease being treated, the severity of the adverse drug reaction, and availability of alternative therapy[see Dosage and Administration ]. - Gastrointestinal Toxicity [see Warnings and Precautions ()]
5.4
Gastrointestinal ToxicityDiarrhea, vomiting, nausea, and stomatitis occurred in up to 10% of patients receiving TREXALL for treatment of non-neoplastic diseases. Hemorrhagic enteritis and fatal intestinal perforation have been reported[see Adverse Reactions ]. Patients with peptic ulcer disease or ulcerative colitis are at a greater risk of developing severe gastrointestinal adverse reactions[see Drug Interactions ].Withhold or discontinue TREXALL for severe gastrointestinal toxicity taking into account the importance of TREXALL treatment in the context of the severity of the disease being treated, the severity of the adverse drug reaction, and availability of alternative therapy[see Dosage and Administration ]. - Hepatotoxicity [see Warnings and Precautions ()]
5.5
HepatotoxicityTREXALL can cause severe and potentially irreversible hepatotoxicity, including fibrosis, cirrhosis, and fatal liver failure
[see Adverse Reactions ]. The safety of TREXALL in patients with hepatic disease is unknown.The risk of hepatotoxicity is increased with heavy alcohol consumption. In patients with psoriasis, fibrosis or cirrhosis may occur in the absence of symptoms or abnormal liver tests; the risk of hepatotoxicity appears to increase with total cumulative dose and generally occurs after receipt of a total cumulative dose of 1.5 g or more.
Monitor liver tests at baseline, periodically during treatment and as clinically indicated. Withhold or discontinue TREXALL taking into account the importance of TREXALL treatment in the context of the severity of the disease being treated, the severity of the adverse drug reaction, and availability of alternative therapy
[see Dosage and Administration ].Monitor patients for pulmonary toxicity and withhold or discontinue methotrexate taking into account the importance of TREXALL treatment in the context of the severity of the disease being treated, the severity of the adverse drug reaction, and availability of alternative therapy[see Dosage and Administration ].
Dermatologic Reactions
Renal Toxicity
Monitor renal function at baseline, periodically during treatment and as clinically indicated. Withhold or discontinue TREXALL for severe renal toxicity taking into account the importa
Administer glucarpidase in patients with toxic plasma methotrexate concentrations (> 1 micromole per liter) and delayed TREXALL clearance due to impaired renal function. Refer to the glucarpidase prescribing information for additional information.
Serious Infections
Monitor patients for infection during and after treatment with methotrexate. Withhold or discontinue methotrexate for serious infections taking into account the importance of methotrexate treatment in the context of the severity of the disease being treated, the severity of the adverse drug reaction, and availability of alternative
Secondary Malignancies
In some cases, lymphoproliferative disease occurring during therapy with low-dose TREXALL regressed completely following withdrawal of methotrexate. If lymphoproliferative d
Increased Risk of Adverse Reactions Due to Third-Space Accumulation
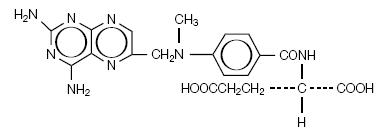
TREXALL® (methotrexate tablets, USP) is dihydrofolate reductase inhibitor with the chemical name of L-glutamic acid,
TREXALL (methotrexate tablets, USP) for oral use is available in bottles of 30 tablets. Each tablet contains 5 mg methotrexate, USP (equivalent to 5.48 mg methotrexate sodium), 7.5 mg methotrexate, USP (equivalent to 8.23 mg methotrexate sodium), 10 mg methotrexate, USP (equivalent to 10.97 mg methotrexate sodium), or 15 mg methotrexate, USP (equivalent to 16.45 mg methotrexate sodium).
The 5 mg also contains: D&C Yellow No. 10 Aluminum lake, FD&C Blue No. 1 Aluminum lake and FD&C Yellow No. 6 Aluminum lake.
The 7.5 mg also contains: FD&C Blue No.1 Aluminum lake.
The 10 mg also contains: FD&C Red No. 40 Aluminum lake.
The 15 mg also contains: FD&C Blue No. 2 Aluminum lake and FD&C Red No. 40 Aluminum lake.
Methotrexate inhibits dihydrofolic acid reductase. Dihydrofolates must be reduced to tetrahydrofolates by this enzyme before they can be utilized as carriers of one-carbon groups in the synthesis of purine nucleotides and thymidylate. Therefore, methotrexate interferes with DNA synthesis, repair, and cellular replication. Actively proliferating tissues such as malignant cells, bone marrow, fetal cells, buccal and intestinal mucosa, and cells of the urinary bladder are in general more sensitive to this effect of methotrexate.
The mechanism of action in rheumatoid arthritis and in psoriasis is unknown.