Triamcinolone Acetonide
Triamcinolone Acetonide Prescribing Information
Triamcinolone Acetonide Ointment is indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid responsive dermatoses.
Apply a thin film of Triamcinolone Acetonide Ointment 0.025% to the affected area two to four times daily.
Apply a thin film of the 0.1% or the 0.5% Triamcinolone Acetonide Ointment, as appropriate, to the affected area two to three times daily.
Occlusive dressings may be used for the management of psoriasis or other recalcitrant conditions. Apply a thin film of ointment to the lesion, cover with a pliable nonporous film, and seal the edges. If needed, additional moisture may be provided by covering the lesion with a dampened clean cotton cloth before the nonporous film is applied or by briefly wetting the affected area with water immediately prior to applying the medication.
The frequency of changing dressings is best determined on an individual basis. It may be convenient to apply Triamcinolone Acetonide Ointment under an occlusive dressing in the evening and to remove the dressing in the morning (i.e., 12-hour occlusion). When utilizing the 12-hour occlusion regimen, additional ointment should be applied, without occlusion, during the day. Reapplication is essential at each dressing change.
If an infection develops, the use of occlusive dressings should be discontinued and appropriate antimicrobial therapy instituted.
Topical corticosteroids are contraindicated in those patients with a history of hypersensitivity to any of the components of the preparations.
The following local adverse reactions are reported infrequently with topical corticosteroids, but may occur more frequently with the use of occlusive dressings (reactions are listed in an approximate decreasing order of occurrence): burning, itching, irritation, dryness, folliculitis, hypertrichosis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, maceration of the skin, secondary infection, skin atrophy, striae, and miliaria.
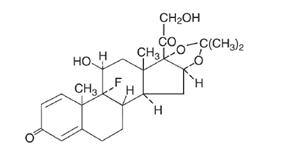
The topical corticosteroids constitute a class of primarily synthetic steroids used as anti-inflammatory and antipruritic agents. The steroids in this class include triamcinolone acetonide. Triamcinolone acetonide is designated chemically as 9-Fluoro-11β, 16α, 17, 21-tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with acetone.
The structural formula is:
Each gram of Triamcinolone Acetonide Ointment USP, 0.025%, 0.1% or 0.5% contains 0.25 mg, 1 mg or 5 mg triamcinolone acetonide, respectively, in an ointment base of light mineral oil and white petrolatum.
Topical corticosteroids share anti-inflammatory, antipruritic and vasoconstrictive actions. The mechanism of anti-inflammatory activity of the topical corticosteroids is unclear. Various laboratory methods, including vasoconstrictor assays, are used to compare and predict potencies and/or clinical efficacies of the topical corticosteroids. There is some evidence to suggest that a recognizable correlation exists between vasoconstrictor potency and therapeutic efficacy in man.
Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids.
Corticosteroids are bound to plasma proteins in varying degrees. Corticosteroids are metabolized primarily in the liver and are then excreted by the kidneys. Some of the topical corticosteroids and their metabolites are also excreted into the bile.