Triamcinolone Acetonide
Triamcinolone Acetonide Prescribing Information
Where oral therapy is not feasible, injectable corticosteroid therapy, including triamcinolone acetonide injectable suspension is indicated
The initial dose of triamcinolone acetonide injectable suspension may vary from 2.5 mg to 100 mg per day depending on the specific disease entity being treated (see
Triamcinolone acetonide injectable suspension is contraindicated in patients who are hypersensitive to any components of this product (see
Intramuscular corticosteroid preparations are contraindicated for idiopathic thrombocytopenic purpura.
The following adverse reactions may be associated with corticosteroid therapy:
Administration
Serious neurologic events, some resulting in death, have been reported with epidural injection of corticosteroids (see
Exposure to excessive amounts of benzyl alcohol has been associated with toxicity (hypotension, metabolic acidosis), particularly in neonates, and an increased incidence of kernicterus, particularly in small preterm infants. There have been rare reports of deaths, primarily in preterm infants, associated with exposure to excessive amounts of benzyl alcohol. The amount of benzyl alcohol from medications is usually considered negligible compared to that received in flush solutions containing benzyl alcohol. Administration of high dosages of medications containing this preservative must take into account the total amount of benzyl alcohol administered. The amount of benzyl alcohol at which toxicity may occur is not known. If the patient requires more than the recommended dosages or other medications containing this preservative, the practitioner must consider the daily metabolic load of benzyl alcohol from these combined sources (see
Rare instances of anaphylaxis have occurred in patients receiving corticosteroid therapy (see
Because triamcinolone acetonide injectable suspension is a suspension, it should
Unless a
Increased dosage of rapidly acting corticosteroids is indicated in patients on corticosteroid therapy subjected to any unusual stress before, during, and after the stressful situation. Triamcinolone acetonide injectable suspension is a long-acting preparation, and is not suitable for use in acute stress situations. To avoid drug-induced adrenal insufficiency, supportive dosage may be required in times of stress (such as trauma, surgery, or severe illness) both during treatment with triamcinolone acetonide injectable suspension and for a year afterwards.
Results from one multicenter, randomized, placebo-controlled study with methylprednisolone hemisuccinate, an intravenous corticosteroid, showed an increase in early (at 2 weeks) and late (at 6 months) mortality in patients with cranial trauma who were determined not to have other clear indications for corticosteroid treatment. High doses of systemic corticosteroids, including triamcinolone acetonide injectable suspension should not be used for the treatment of traumatic brain injury.
Average and large doses of corticosteroids can cause elevation of blood pressure, salt and water retention and increased excretion of potassium. These effects are less likely to occur with the synthetic derivatives except when they are used in large doses. Dietary salt restriction and potassium supplementation may be necessary (see
Literature reports suggest an apparent association between use of corticosteroids and left ventricular free wall rupture after a recent myocardial infarction; therefore, therapy with corticosteroids should be used with great caution in these patients.
There have been cases reported in which concomitant use of amphotericin B and hydrocortisone was followed by cardiac enlargement and congestive heart failure (see
Corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency after withdrawal of treatment.
Metabolic clearance of corticosteroids is decreased in hypothyroid patients and increased in hyperthyroid patients. Changes in thyroid status of the patient may necessitate adjustment in dosage.
Corticosteroids, including triamcinolone acetonide, suppress the immune system and increase the risk of infection with any pathogen, including viral, bacterial, fungal, protozoan, or helminthic pathogens. Corticosteroids can:
- Reduce resistance to new infections
- Exacerbate existing infections
- Increase the risk of disseminated infections
- Increase the risk of reactivation or exacerbation of latent infections
- Mask some signs of infection
Corticosteroid-associated infections can be mild but can be severe and at times fatal. The rate of infectious complications increases with increasing corticosteroid dosages.
Monitor for the development of infection and consider triamcinolone acetonide withdrawal or dosage reduction as needed.
Do not administer triamcinolone acetonide injectable suspension by an intraarticular, intrabursal, or intratendinous route in the presence of acute local infection.
If triamcinolone acetonide is used to treat a condition in patients with latent tuberculosis or tuberculin reactivity, reactivation of the disease may occur. Closely monitor such patients for reactivation. During prolonged triamcinolone acetonide therapy, patients with latent tuberculosis or tuberculin reactivity should receive chemoprophylaxis.
Varicella and measles can have a serious or even fatal course in non-immune patients receiving corticosteroids, including triamcinolone acetonide. In corticosteroid-treated patients who have not had these diseases or are non-immune, particular care should be taken to avoid exposure to varicella and measles:
- If a triamcinolone acetonide-treated patient is exposed to varicella, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If varicella develops, treatment with antiviral agents may be considered.
- If a triamcinolone acetonide-treated patient is exposed to measles, prophylaxis with immunoglobulin (IG) may be indicated.
Hepatitis B virus reactivation can occur in patients who are hepatitis B carriers treated with immunosuppressive dosages of corticosteroids, including triamcinolone acetonide. Reactivation can also occur infrequently in corticosteroid-treated patients who appear to have resolved hepatitis B infection.
Screen patients for hepatitis B infection before initiating immunosuppressive (e.g., prolonged) treatment with triamcinolone acetonide. For patients who show evidence of hepatitis B infection, recommend consultation with physicians with expertise in managing hepatitis B regarding monitoring and consideration for hepatitis B antiviral therapy.
Corticosteroids, including triamcinolone acetonide, may exacerbate systemic fungal infections; therefore, avoid triamcinolone acetonide use in the presence of such infections unless triamcinolone acetonide is needed to control drug reactions. For patients on chronic triamcinolone acetonide therapy who develop systemic fungal infections, triamcinolone acetonide withdrawal or dosage reduction is recommended.
Corticosteroids, including triamcinolone acetonide, may activate latent amebiasis. Therefore, it is recommended that latent amebiasis or active amebiasis be ruled out before initiating triamcinolone acetonide in patients who have spent time in the tropics or patients with unexplained diarrhea.
Corticosteroids, including triamcinolone acetonide, should be used with great care in patients with known or suspected Strongyloides (threadworm) infestation. In such patients, corticosteroid-induced immunosuppression may lead to Strongyloides hyperinfection and dissemination with widespread larval migration, often accompanied by severe enterocolitis and potentially fatal gram-negative septicemia.
Avoid corticosteroids, including triamcinolone acetonide, in patients with cerebral malaria.
Epidural and intrathecal administration of this product is not recommended. Reports of serious medical events, including death, have been associated with epidural and intrathecal routes of corticosteroid administration (see
Use of corticosteroids may produce posterior subcapsular cataracts, glaucoma with possible damage to the optic nerves, and may enhance the establishment of secondary ocular infections due to bacteria, fungi, or viruses. The use of oral corticosteroids is not recommended in the treatment of optic neuritis and may lead to an increase in the risk of new episodes. Corticosteroids should not be used in active ocular herpes simplex.
Adequate studies to demonstrate the safety of triamcinolone acetonide injectable suspension use by intraturbinal, subconjunctival, sub-Tenons, retrobulbar and intraocular (intravitreal) injections have not been performed. Endophthalmitis, eye inflammation, increased intraocular pressure and visual disturbances including vision loss have been reported with intravitreal administration. Administration of triamcinolone acetonide injectable suspension intraocularly or into the nasal turbinates is not recommended.
Intraocular injection of corticosteroid formulations containing benzyl alcohol, such as triamcinolone acetonide injectable suspension is not recommended because of potential toxicity from the benzyl alcohol.
Kaposi’s sarcoma has been reported to occur in patients receiving corticosteroid therapy, most often for chronic conditions. Discontinuation of corticosteroids may result in clinical improvement of Kaposi’s sarcoma.
Triamcinolone acetonide injectable suspension, USP is a synthetic glucocorticoid corticosteroid with anti-inflammatory action. THIS FORMULATION IS SUITABLE FOR INTRAMUSCULAR AND INTRA-ARTICULAR USE ONLY. THIS FORMULATION IS NOT FOR INTRADERMAL INJECTION.
Each mL of the sterile aqueous suspension provides 40 mg triamcinolone acetonide, USP, with 0.65% sodium chloride for isotonicity, 0.99% (w/v) benzyl alcohol as a preservative, 0.75% carboxymethylcellulose sodium, and 0.04% polysorbate 80 in an aqueous suspension. Sodium hydroxide or hydrochloric acid may be present to adjust pH to 5.0 to 7.5. At the time of manufacture, the air in the container is replaced by nitrogen.
The chemical name for triamcinolone acetonide is 9-Fluoro-11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with acetone. Its structural formula is:
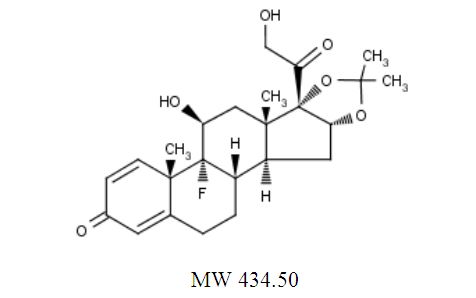
Triamcinolone acetonide, USP occurs as a white to cream-colored, crystalline powder having not more than a slight odor and is practically insoluble in water and very soluble in alcohol.