Triamterene And Hydrochlorothiazide
Triamterene And Hydrochlorothiazide Prescribing Information
- Triamterene and hydrochlorothiazide tablets are indicated for the treatment of hypertension or edema in patients who develop hypokalemia on hydrochlorothiazide alone.
- Triamterene and hydrochlorothiazide tablets are also indicated for those patients who require a thiazide diuretic and in whom the development of hypokalemia cannot be risked (e.g., patients on concomitant digitalis preparations, or with a history of cardiac arrhythmias, etc.).
Triamterene and hydrochlorothiazide tablets may be used alone or in combination with other antihypertensive drugs, such as beta-blockers. Since triamterene and hydrochlorothiazide may enhance the actions of these drugs, dosage adjustments may be necessary.
Note: 37.5 mg/25 mg=37.5 mg triamterene and 25 mg hydrochlorothiazide
75 mg/50 mg=75 mg triamterene and 50 mg hydrochlorothiazide
The usual dose of triamterene and hydrochlorothiazide 37.5 mg/25 mg, is one or two tablets daily, given as a single dose, with appropriate monitoring of serum potassium (see
Patients receiving 50 mg of hydrochlorothiazide who become hypokalemic may be transferred to the 75 mg/50 mg product, directly. Patients receiving 25 mg hydrochlorothiazide who become hypokalemic may be transferred to the 37.5 mg/25 mg product, directly.
In patients requiring hydrochlorothiazide therapy and in whom hypokalemia cannot be risked, therapy may be initiated with 37.5 mg/25 mg of triamterene and hydrochlorothiazide. If an optimal blood pressure response is not obtained with 37.5 mg/25 mg of triamterene and hydrochlorothiazide, the dose should be increased to two 37.5 mg/25 mg tablets daily as a single dose, or one 75 mg/50 mg tablet daily. If blood pressure still is not controlled, another antihypertensive agent may be added (see
Clinical studies have shown that patients taking less bioavailable formulations of triamterene and hydrochlorothiazide in daily doses of 25 mg to 50 mg of hydrochlorothiazide and 50 mg to 100 mg of triamterene may be safely changed to one triamterene and hydrochlorothiazide 37.5 mg/25 mg tablet daily. All patients changed from less bioavailable formulations to triamterene and hydrochlorothiazide tablets should be monitored clinically and for serum potassium after the transfer.
Side effects observed in association with the use of triamterene and hydrochlorothiazide tablets, other combination products containing triamterene and hydrochlorothiazide, and products containing triamterene or hydrochlorothiazide include the following:
Whenever adverse reactions are moderate to severe, therapy should be reduced or withdrawn.
Thiazides may add to or potentiate the action of other antihypertensive drugs.
The thiazides may decrease arterial responsiveness to norepinephrine. This diminution is not sufficient to preclude effectiveness of the pressor agent for therapeutic use. Thiazides have also been shown to increase the responsiveness to tubocurarine.
Lithium generally should not be given with diuretics because they reduce its renal clearance and add a high risk of lithium toxicity. Refer to the package insert on lithium before use of such concomitant therapy.
Acute renal failure has been reported in a few patients receiving indomethacin and formulations containing triamterene and hydrochlorothiazide. Caution is therefore advised when administering non-steroidal anti-inflammatory agents with triamterene and hydrochlorothiazide.
Potassium-sparing agents should be used very cautiously, if at all, in conjunction with angiotensin-converting enzyme (ACE) inhibitors due to a greatly increased risk of hyperkalemia. Serum potassium should be monitored frequently.
Triamterene and hydrochlorothiazide tablets, USP combine triamterene USP, a potassium-conserving diuretic, with the natriuretic agent, hydrochlorothiazide, USP. Triamterene and hydrochlorothiazide tablets, USP are available in two strengths. Each triamterene and hydrochlorothiazide tablet USP, 75 mg/50 mg, contains triamterene USP, 75 mg and hydrochlorothiazide USP, 50 mg. Each triamterene and hydrochlorothiazide tablet USP,
37.5 mg/25 mg, contains triamterene USP, 37.5 mg and hydrochlorothiazide USP, 25 mg.
Both strengths of triamterene and hydrochlorothiazide tablets, USP for oral administration contain the following inactive ingredients: anhydrous lactose, magnesium stearate, microcrystalline cellulose, polacrilin potassium, polyethylene glycol 8000, and povidone. Triamterene and hydrochlorothiazide tablets, 37.5 mg/25 mg also contain FD&C Blue No. 2 Aluminum Lake.
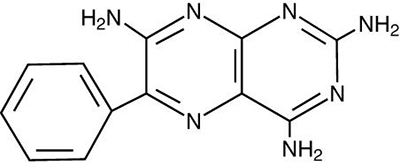
Triamterene, USP is 2,4,7-triamino-6-phenylpteridine. Triamterene, USP is practically insoluble in water, benzene, chloroform, ether and dilute alkali hydroxides. It is soluble in formic acid and sparingly soluble in methoxyethanol. Triamterene, USP is very slightly soluble in acetic acid, alcohol and dilute mineral acids. Its molecular weight is 253.27. Its structural formula is:
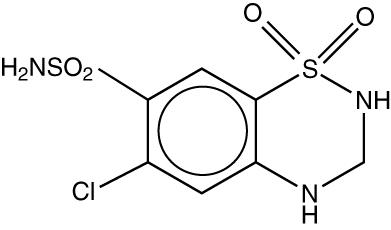
Hydrochlorothiazide, USP is 6-chloro-3,4-dihydro-2
Triamterene and hydrochlorothiazide is a diuretic, antihypertensive drug product, principally due to its hydrochlorothiazide component; the triamterene component reduces the excessive potassium loss which may occur with hydrochlorothiazide use.