Ustekinumab - Ttwe - Ustekinumab - Ttwe injection, Solution
(Ustekinumab-Ttwe)Ustekinumab - Ttwe - Ustekinumab - Ttwe injection, Solution Prescribing Information
Ustekinumab-ttwe (ustekinumab-ttwe) is a clear, colorless to light yellow, sterile and preservative-free solution.
- Injection: 45 mg/0.5 mL or 90 mg/mL solution in a single-dose prefilled syringe
- Injection: 130 mg/26 mL (5 mg/mL) solution in a single-dose vial
Ustekinumab-ttwe is contraindicated in patients with clinically significant hypersensitivity to ustekinumab products or to any of the excipients in Ustekinumab-ttwe
Hypersensitivity reactions, including anaphylaxis and angioedema, have been reported with ustekinumab products
The following serious adverse reactions are discussed elsewhere in the label:
- Infections [see Warnings and Precautions ()]
5.1 InfectionsUstekinumab products may increase the risk of infections and reactivation of latent infections. Serious bacterial, mycobacterial, fungal, and viral infections were observed in patients receiving ustekinumab products
[see Adverse Reactions ].Serious infections requiring hospitalization, or otherwise clinically significant infections, reported in clinical trials included the following:
- Plaque Psoriasis:diverticulitis, cellulitis, pneumonia, appendicitis, cholecystitis, sepsis, osteomyelitis, viral infections, gastroenteritis and urinary tract infections.
- Psoriatic arthritis:cholecystitis.
- Crohn's disease:anal abscess, gastroenteritis, ophthalmic herpes zoster, pneumonia, and listeria meningitis.
- Ulcerative colitis:gastroenteritis, ophthalmic herpes zoster, pneumonia, and listeriosis.
Avoid initiating treatment with Ustekinumab-ttwe in patients with any clinically important active infection until the infection resolves or is adequately treated. Consider the risks and benefits of treatment prior to initiating use of Ustekinumab-ttwe in patients with a chronic infection or a history of recurrent infection.
Instruct patients to seek medical advice if signs or symptoms suggestive of an infection occur while on treatment with Ustekinumab-ttwe and discontinue Ustekinumab-ttwe for serious or clinically significant infections until the infection resolves or is adequately treated.
- Malignancies [see Warnings and Precautions ()]
5.4 MalignanciesUstekinumab products are immunosuppressants and may increase the risk of malignancy. Malignancies were reported among subjects who received ustekinumab in clinical trials
[see Adverse Reactions ]. In rodent models, inhibition of IL-12/IL-23p40 increased the risk of malignancy[see Nonclinical Toxicology ].The safety of ustekinumab products has not been evaluated in patients who have a history of malignancy or who have a known malignancy.
There have been post-marketing reports of the rapid appearance of multiple cutaneous squamous cell carcinomas in patients receiving ustekinumab products who had pre-existing risk factors for developing non-melanoma skin cancer. Monitor all patients receiving Ustekinumab-ttwe for the appearance of non-melanoma skin cancer. Closely follow patients greater than 60 years of age, those with a medical history of prolonged immunosuppressant therapy and those with a history of PUVA treatment[see Adverse Reactions ]. - Hypersensitivity Reactions [see Warnings and Precautions ()]
5.5 Hypersensitivity ReactionsHypersensitivity reactions, including anaphylaxis and angioedema, have been reported with ustekinumab products
[see Adverse Reactions ]. If an anaphylactic or other clinically significant hypersensitivity reaction occurs, institute appropriate therapy and discontinue Ustekinumab-ttwe. - Posterior Reversible Encephalopathy Syndrome (PRES) [see Warnings and Precautions ()]
5.6 Posterior Reversible Encephalopathy Syndrome (PRES)Two cases of posterior reversible encephalopathy syndrome (PRES), also known as Reversible Posterior Leukoencephalopathy Syndrome (RPLS), were reported in clinical trials. Cases have also been reported in postmarketing experience in patients with psoriasis, psoriatic arthritis and Crohn's disease. Clinical presentation included headaches, seizures, confusion, visual disturbances, and imaging changes consistent with PRES a few days to several months after ustekinumab product initiation. A few cases reported latency of a year or longer. Patients recovered with supportive care following withdrawal of ustekinumab products.
Monitor all patients treated with Ustekinumab-ttwe for signs and symptoms of PRES. If PRES is suspected, promptly administer appropriate treatment and discontinue Ustekinumab-ttwe.
- Noninfectious Pneumonia [see Warnings and Precautions ()]
5.8 Noninfectious PneumoniaCases of interstitial pneumonia, eosinophilic pneumonia and cryptogenic organizing pneumonia have been reported during post-approval use of ustekinumab products. Clinical presentations included cough, dyspnea, and interstitial infiltrates following one to three doses. Serious outcomes have included respiratory failure and prolonged hospitalization. Patients improved with discontinuation of therapy and in certain cases administration of corticosteroids. If diagnosis is confirmed, discontinue Ustekinumab-ttwe and institute appropriate treatment
[see Adverse Reactions ].
Ustekinumab-ttwe, a human IgG1κ monoclonal antibody, is a human interleukin-12 and -23 antagonist. Using DNA recombinant technology, ustekinumab-ttwe is produced in a Chinese hamster ovary cell line. The manufacturing process contains steps for the clearance of viruses. Ustekinumab-ttwe is comprised of 1326 amino acids and has an estimated molecular mass that ranges from 148,079 to 149,690 Daltons.
Ustekinumab-ttwe (ustekinumab-ttwe) injection is a clear, colorless to light yellow, sterile and preservative-free solution with pH of 5.7– 6.3.
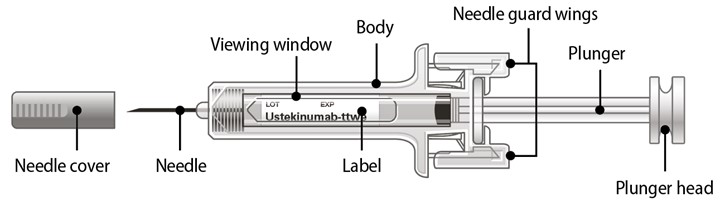
Available as 45 mg of ustekinumab-ttwe in 0.5 mL and 90 mg of ustekinumab-ttwe in 1 mL, supplied as a sterile solution in a single-dose prefilled syringe with a 29 gauge fixed ½ inch needle. The syringe is fitted with a passive needle guard and a needle cover.
Each 0.5 mL prefilled syringe delivers 45 mg ustekinumab-ttwe, histidine (0.095 mg), histidine hydrochloride monohydrate (0.405 mg), polysorbate 80 (0.02 mg), and sucrose (42.5 mg).
Each 1 mL prefilled syringe delivers 90 mg ustekinumab-ttwe, histidine (0.19 mg), histidine hydrochloride monohydrate (0.81 mg), polysorbate 80 (0.04 mg), and sucrose (85 mg).
Available as 130 mg of ustekinumab-ttwe in 26 mL, supplied as a single-dose 30 mL Type I glass vial with a coated stopper.
Each 26 mL vial delivers 130 mg ustekinumab-ttwe, edetate disodium (0.52 mg), histidine (20 mg), histidine hydrochloride monohydrate (27 mg), methionine (10.4 mg), polysorbate 80 (10.4 mg) and sucrose (2,210 mg).
Ustekinumab-ttwe (ustekinumab-ttwe) injection is a clear, colorless to light yellow, sterile and preservative-free solution. It is supplied as individually packaged, single-dose prefilled syringes or single-dose vials.
- 45 mg/0.5 mL (NDC 82009-160-11)
- 90 mg/mL (NDC 82009-162-11)
Each prefilled syringe is equipped with a 29 gauge fixed ½ inch needle, a needle safety guard, and a needle cover that is not made with natural rubber latex.
• 130 mg/26 mL (5 mg/mL) (NDC 82009-163-94)
Store Ustekinumab-ttwe vials and prefilled syringes refrigerated between 2°C to 8°C (36°F to 46°F). Store Ustekinumab-ttwe vials upright. Keep the product in the original carton to protect from light until the time of use. Do not freeze. Do not shake.
If needed, individual prefilled syringes may be stored at room temperature up to 30°C (86°F) for a maximum single period of up to 35 days in the original carton to protect from light. If not used within 35 days of room temperature storage, discard the prefilled syringe. The prefilled syringe may be returned to the refrigerator one time only for a maximum of 60 days. If not used within 60 days, discard the prefilled syringe. Record the date when the prefilled syringe is removed from and returned to the refrigerator on the carton in the space provided.
Do not use Ustekinumab-ttwe after the expiration date on the carton or on the prefilled syringe.
If you cannot give yourself the injection:
• ask your doctor or nurse to help you, or
• ask someone who has been trained by a doctor or nurse to give your injections.
Do not try to inject Ustekinumab-ttwe yourself until you have been shown how to inject Ustekinumab-ttwe by your doctor, nurse or health professional.
- Before you start, check the carton to make sure that it is the right dose. You will have either 45 mg or 90 mg as prescribed by your doctor.
- If your dose is 45 mg, you will receive one 45 mg prefilled syringe.
- If your dose is 90 mg, you will receive either one 90 mg prefilled syringe or two 45 mg prefilled syringes. If you receive two 45 mg prefilled syringes for a 90 mg dose, you will need to give yourself two injections, one right after the other.
- Children 12 years of age and older with psoriasis who weigh 132 pounds (60 kg) or more may use a prefilled syringe.
- Check the expiration date on the prefilled syringe and carton. Do notuse Ustekinumab-ttwe after the expiration date has passed. If the expiration date has passed or if the prefilled syringe has been stored above 86ºF (30ºC), call your doctor or pharmacist, or call 1-877-605-7243 for help.
- Make sure the syringe is not damaged. Do not use the prefilled syringe if it is damaged.
- Check your prefilled syringe for any particles or discoloration. Your prefilled syringe should look clear and colorless to light yellow.
- Do not use if it is frozen, discolored, cloudy or has particles. Get a new prefilled syringe.
- Do not shake the prefilled syringe at any time.Shaking your prefilled syringe may damage your Ustekinumab-ttwe medicine. If your prefilled syringe has been shaken, do not use it. Get a new prefilled syringe.
- To reduce the risk of accidental needle sticks, each prefilled syringe has a needle guard that is automatically activated to cover the needle after you have given your injection. Do not pull back on the plunger at any time.
- Store Ustekinumab-ttwe in a refrigerator between 36°F to 46°F (2°C to 8°C).
- Store Ustekinumab-ttwe in the original carton to protect from light until the time of use.
- Do not freeze Ustekinumab-ttwe.
- If needed, individual prefilled syringes may be stored at room temperature up to 86ºF (30ºC) for a maximum single period of up to 35 days in the original carton to protect from light.
- Record the date when the prefilled syringe is removed from the refrigerator on the carton in the space provided.
- You may return the prefilled syringe to the refrigerator 1 time only for a maximum of 60 days, either during the 35-day period or at the end of the 35-day period.
- Record the date when the prefilled syringe is returned to the refrigerator on the carton.
- Discard the prefilled syringe if not used within 35 days of room temperature storage and you did not return it to the refrigerator, or if it has been returned to the refrigerator and is not used within 60 days.
You will need:
- antiseptic wipes
- cotton balls or gauze pads
- adhesive bandage
- your prescribed dose of Ustekinumab-ttwe (See Figure B)
- FDA-cleared sharps disposal container. See "Step 4: Dispose of the syringe."

- Choose a well-lit, clean, flat work surface.
- Leave Ustekinumab-ttwe prefilled syringe at room temperature for about 30 minutes before injecting. Do not warm the prefilled syringe in any other way (for example, do not warm it in a microwave or in hot water).
- Wash your hands well with soap and warm water.
- Hold the prefilled syringe with the covered needle pointing upward.
- Choose an injection site around your stomach area (abdomen), buttocks, upper legs (thighs). If a caregiver is giving you the injection, the outer area of the upper arms may also be used. (See Figure C)
- Use a different injection site for each injection.Do notgive an injection in an area of the skin that is tender, bruised, red or hard.
- Clean the skin with an antiseptic wipe where you plan to give your injection.
- Do nottouch this area again before giving the injection. Let your skin dry before injecting.
- Do notfan or blow on the clean area.
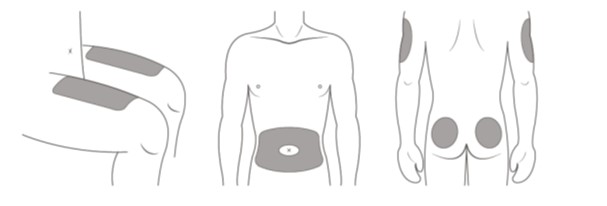
*Areas in gray are recommended injection sites.
Step 3: Inject Ustekinumab-ttwe
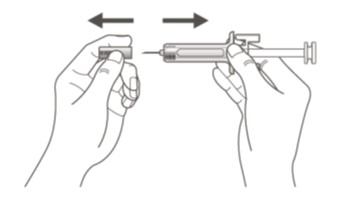
- Remove the needle cover when you are ready to inject your Ustekinumab-ttwe.
- Do nottouch the plunger or plunger head while removing the needle cover.
- Hold the body of the prefilled syringe with one hand, and pull the needle cover straight off. (See Figure D)
- Put the needle cover in the trash.
- You may also see a drop of liquid at the end of the needle. This is normal.
- Do nottouch the needle or let it touch anything.
- Do notuse the prefilled syringe if it is dropped without the needle cover in place. Call your doctor, nurse or health professional for instructions.
• Hold the body of the prefilled syringe in one hand between the thumb and index fingers.
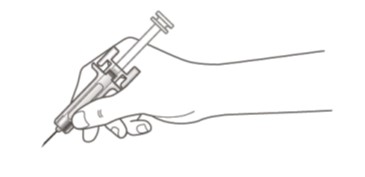
- Do notpull back on the plunger at any time.
- Use the other hand to gently pinch the cleaned area of skin. Hold firmly.
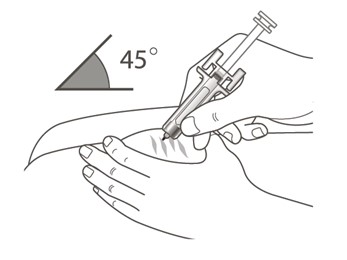
- Use a quick, dart-like motion to insert the needle into the pinched skin at about a 45-degree angle. (See Figure F)
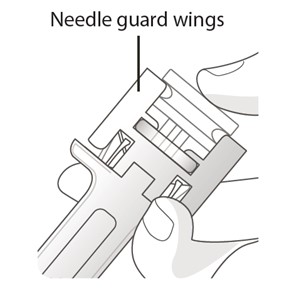
- Inject all of the liquid by using your thumb to push in the plunger until the plunger head is completely between the needle guard wings. (See Figure G)
- When the plunger is pushed as far as it will go, keep pressure on the plunger head. Take the needle out of the skin and let go of the skin.
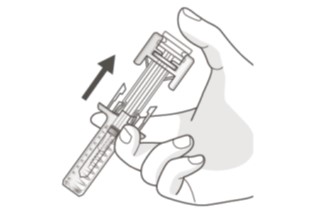
- Slowly take your thumb off the plunger head. This will let the empty syringe move up until the entire needle is covered by the needle guard. (See Figure H)
- When the needle is pulled out of your skin, there may be a little bleeding at the injection site. This is normal. You can press a cotton ball or gauze pad to the injection site if needed. Do not rub the injection site. You may cover the injection site with a small adhesive bandage, if necessary.
- Put the syringe in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose syringes in your household trash.
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant,
- and properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be local or state laws about how to throw away syringes and needles. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your sharps disposal container.
- If you have any questions, talk to your doctor or pharmacist.
Manufactured by:
Samsung Bioepis Co., Ltd.,
76, Songdogyoyuk-ro, Yeonsu-gu, Incheon, 21987, Republic of Korea
U.S. License No. 2046
Manufactured for:
Quallent Pharmaceuticals Health LLC,
Grand Cayman, Cayman Islands
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Revised 03/2025