Varenicline
(Varenicline Tartrate)Varenicline Prescribing Information
Varenicline tablets are indicated for use as an aid to smoking cessation treatment.
Circular, biconvex tablets: 0.5 mg (white to off-white film-coated tablets, debossed with “P” on one side and “155” on other side) and 1 mg (light blue film-coated tablets, debossed with “P” on one side and “156” on other side).
Varenicline tablets are contraindicated in patients with a known history of serious hypersensitivity reactions or skin reactions to varenicline tablets.
The following serious adverse reactions were reported in postmarketing experience and are discussed in greater detail in other sections of the labeling:
- Neuropsychiatric Adverse Events including Suicidality [see Warnings and Precautions (5.1)]
- Seizures [see Warnings and Precautions (5.2)]
- Interaction with Alcohol [see Warnings and Precautions (5.3)]
- Accidental Injury [see Warnings and Precautions (5.4)]
- Cardiovascular Events [see Warnings and Precautions (5.5)]
- Somnambulism [see Warnings and Precautions (5.6)]
- Angioedema and Hypersensitivity Reactions [see Warnings and Precautions (5.7)]
- Serious Skin Reactions [see Warnings and Precautions (5.8)]
In the placebo-controlled premarketing studies, the most common adverse events associated with varenicline (>5% and twice the rate seen in placebo-treated patients) were nausea, abnormal (vivid, unusual, or strange) dreams, constipation, flatulence, and vomiting.
The treatment discontinuation rate due to adverse events in patients dosed with 1 mg twice daily was 12% for varenicline, compared to 10% for placebo in studies of three months’ treatment. In this group, the discontinuation rates that are higher than placebo for the most common adverse events in varenicline-treated patients were as follows: nausea (3% vs. 0.5% for placebo), insomnia (1.2% vs. 1.1% for placebo), and abnormal dreams (0.3% vs. 0.2% for placebo).
Smoking cessation, with or without treatment, is associated with nicotine withdrawal symptoms and has also been associated with the exacerbation of underlying psychiatric illness.
Based on varenicline characteristics and clinical experience to date, varenicline has no clinically meaningful pharmacokinetic drug interactions
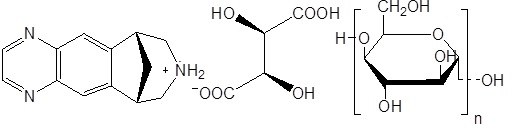
Varenicline tablets contain varenicline (as the tartrate salt), which is a partial nicotinic agonist selective for α4β2 nicotinic acetylcholine receptor subtypes.
Varenicline, as the tartrate salt of maltodextrin premix (1:1:10), is a powder which is a off-white to pinkish brown color with the following chemical name: 7,8,9,10-tetrahydro-6,10-methano-6
Varenicline tablets are supplied for oral administration in two strengths: a 0.5 mg circular, biconvex, white to off-white film-coated tablets, debossed with “P” on one side and “155” on other side and a 1 mg circular, biconvex, light blue film-coated tablets, debossed with “P” on one side and “156” on other side. Each 0.5 mg varenicline tablet contains 0.85 mg of varenicline tartrate equivalent to 0.5 mg of varenicline free base; each 1 mg varenicline tablet contains 1.71 mg of varenicline tartrate equivalent to 1 mg of varenicline free base. The following inactive ingredients are included in the tablets: croscarmellose sodium, maltodextrin, microcrystalline cellulose, stearic acid. The tablets are film-coated with a coating material containing hydroxypropyl cellulose, hypromellose, talc, and titanium dioxide. In addition to these, the 1 mg tablet film coating includes FD&C blue #2/indigo carmine aluminum lake and iron oxide yellow.