Verapamil Hydrochloride
Verapamil Hydrochloride Prescribing Information
Verapamil Hydrochloride Injection, USP is indicated for the following:
- Rapid conversion to sinus rhythm of paroxysmal supraventricular tachycardias, including those associated with accessory bypass tracts (Wolff-Parkinson-White [W-P-W] and Lown-Ganong- Levine [L-G-L] syndromes). When clinically advisable, appropriate vagal maneuvers (e.g., Valsalva maneuver) should be attempted prior to verapamil hydrochloride administration.
- Temporary control of rapid ventricular rate in atrial flutter or atrial fibrillation except when the atrial flutter and/or atrial fibrillation are associated with accessory bypass tracts (Wolff-Parkinson-White (W-P-W) and Lown-Ganong-Levine (L-G-L) syndromes).
In controlled studies in the United States, about 60% of patients with supraventricular tachycardia converted to normal sinus rhythm within 10 minutes after intravenous verapamil hydrochloride. Uncontrolled studies reported in the world literature describe a conversion rate of about 80%. About 70% of patients with atrial flutter and/or fibrillation with a faster ventricular rate respond with a decrease in ventricular rate of at least 20%. Conversion of atrial flutter or fibrillation to sinus rhythm is uncommon (about 10%) after verapamil hydrochloride and may reflect the spontaneous conversion rate, since the conversion rate after placebo was similar. Slowing of the ventricular rate in patients with atrial fibrillation/flutter lasts 30 to 60 minutes after a single injection.
Verapamil hydrochloride injection is contraindicated in:
1. Severe hypotension or cardiogenic shock.
2. Second- or third-degree AV block (except in patients with a functioning artificial ventricular pacemaker).
3. Sick sinus syndrome (except in patients with a functioning artificial ventricular pacemaker).
4. Severe congestive heart failure (unless secondary to a supraventricular tachycardia amenable to verapamil therapy).
5. Patients receiving
6. Patients with atrial flutter or atrial fibrillation and an accessory bypass tract (e.g., Wolff- Parkinson-White, Lown-Ganong-Levine syndromes) are at risk to develop ventricular tachyarrhythmia including ventricular fibrillation if verapamil is administered. Therefore, the use of verapamil in these patients is contraindicated.
7. Ventricular tachycardia: Administration of intravenous verapamil to patients with wide-complex ventricular tachycardia (QRS ≥ 0.12 sec) can result in marked hemodynamic deterioration and ventricular fibrillation. Proper pretherapy diagnosis and differentiation from wide-complex supraventricular tachycardia is imperative in the emergency room setting.
8. Known hypersensitivity to verapamil hydrochloride.
Verapamil cannot be removed by hemodialysis.
The following reactions were reported with verapamil hydrochloride injection used in controlled U.S. clinical trials involving 324 patients:
In rare cases of hypersensitive patients, broncho/laryngeal spasm accompanied by itch and urticaria has been reported.
The following reactions have been reported at low frequency: emotional depression, rotary nystagmus, sleepiness, vertigo, muscle fatigue, diaphoresis, and respiratory failure.
Suggested Treatment of Acute Cardiovascular Adverse Reactions* | ||
| The frequency of these adverse reactions was quite low, and experience with their treatment has been limited. | ||
Adverse Reaction | Proven Effective Treatment | Supportive Treatment |
| 1.Symptomatic hypotension requiring treatment | Dopamine intravenous Calcium chloride intravenous Norepinephrine bitartrate Intravenous Metaraminol bitartrate intravenous Isoproterenol HCl intravenous | Intravenous fluids Trendelenburg position |
| 2. Bradycardia, AV block, Asystole | Isoproterenol HCl intravenous Calcium chloride intravenous Norepinephrine bitartrate intravenous Atropine intravenous Cardiac pacing | Intravenous fluids (slow drip) |
| 3. Rapid ventricular rate (due to antegrade conduction in flutter/fibrillation with W-P-W or L-G-L syndromes) | DC-cardioversion (high energy may be required) Procainamide intravenous Lidocaine intravenous | Intravenous fluids (slow drip) |
Cardioversion has been used safely and effectively after verapamil hydrochloride injection.
For stability reasons this product is not recommended for dilution with Sodium Lactate Injection, USP in polyvinyl chloride bags. Verapamil is physically compatible and chemically stable for at least 24 hours at 25°C protected from light in most common large volume parenteral solutions. Admixing verapamil hydrochloride injection with albumin, amphotericin B, hydralazine hydrochloride and trimethoprim with sulfamethoxazole should be avoided. Verapamil hydrochloride injection will precipitate in any solution with a pH above 6.0.
Verapamil hydrochloride injection is contraindicated in:
1. Severe hypotension or cardiogenic shock.
2. Second- or third-degree AV block (except in patients with a functioning artificial ventricular pacemaker).
3. Sick sinus syndrome (except in patients with a functioning artificial ventricular pacemaker).
4. Severe congestive heart failure (unless secondary to a supraventricular tachycardia amenable to verapamil therapy).
5. Patients receiving
6. Patients with atrial flutter or atrial fibrillation and an accessory bypass tract (e.g., Wolff- Parkinson-White, Lown-Ganong-Levine syndromes) are at risk to develop ventricular tachyarrhythmia including ventricular fibrillation if verapamil is administered. Therefore, the use of verapamil in these patients is contraindicated.
7. Ventricular tachycardia: Administration of intravenous verapamil to patients with wide-complex ventricular tachycardia (QRS ≥ 0.12 sec) can result in marked hemodynamic deterioration and ventricular fibrillation. Proper pretherapy diagnosis and differentiation from wide-complex supraventricular tachycardia is imperative in the emergency room setting.
8. Known hypersensitivity to verapamil hydrochloride.
The following reactions were reported with verapamil hydrochloride injection used in controlled U.S. clinical trials involving 324 patients:
In rare cases of hypersensitive patients, broncho/laryngeal spasm accompanied by itch and urticaria has been reported.
The following reactions have been reported at low frequency: emotional depression, rotary nystagmus, sleepiness, vertigo, muscle fatigue, diaphoresis, and respiratory failure.
Suggested Treatment of Acute Cardiovascular Adverse Reactions* | ||
| The frequency of these adverse reactions was quite low, and experience with their treatment has been limited. | ||
Adverse Reaction | Proven Effective Treatment | Supportive Treatment |
| 1.Symptomatic hypotension requiring treatment | Dopamine intravenous Calcium chloride intravenous Norepinephrine bitartrate Intravenous Metaraminol bitartrate intravenous Isoproterenol HCl intravenous | Intravenous fluids Trendelenburg position |
| 2. Bradycardia, AV block, Asystole | Isoproterenol HCl intravenous Calcium chloride intravenous Norepinephrine bitartrate intravenous Atropine intravenous Cardiac pacing | Intravenous fluids (slow drip) |
| 3. Rapid ventricular rate (due to antegrade conduction in flutter/fibrillation with W-P-W or L-G-L syndromes) | DC-cardioversion (high energy may be required) Procainamide intravenous Lidocaine intravenous | Intravenous fluids (slow drip) |
(See
Verapamil cannot be removed by hemodialysis.
Verapamil hydrochloride is a calcium antagonist or slow-channel inhibitor. Verapamil Hydrochloride Injection, USP is a sterile, nonpyrogenic solution containing verapamil hydrochloride 2.5 mg/mL and sodium chloride 8.5 mg/mL in water for injection. The solution contains no bacteriostat or antimicrobial agent and is intended for single-dose intravenous administration. May contain hydrochloric acid and/or sodium hydroxide for pH adjustment; pH is 4.0 to 6.5.
The chemical name of Verapamil Hydrochloride, USP is benzeneacetonitrile, α-[3-[{2-(3,4-dimethoxyphenyl)ethyl} methylamino] propyl]-3,4-dimethoxy-α-(1-methylethyl) hydrochloride. Verapamil hydrochloride is a white or practically white crystalline powder. It is practically odorless and has a bitter taste. It is soluble in water; freely soluble in chloroform; sparingly soluble in alcohol; practically insoluble in ether. It has the following structural formula:
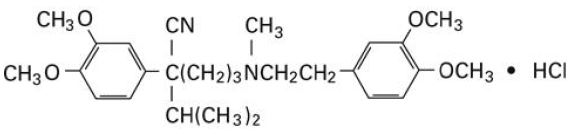
Molecular weight: 491.07
Molecular formula: C27H38N2O4 • HCl
Verapamil hydrochloride is not chemically related to other antiarrhythmic drugs.