Vyloy
(Zolbetuximab)Vyloy Prescribing Information
VYLOY, in combination with fluoropyrimidine- and platinum-containing chemotherapy, is indicated for the first-line treatment of adults with locally advanced unresectable or metastatic human epidermal growth factor receptor 2 (HER2)‑negative gastric or gastroesophageal junction (GEJ) adenocarcinoma whose tumors are claudin (CLDN) 18.2 positive as determined by an FDA-approved test
Select adult patients with locally advanced unresectable or metastatic HER2-negative gastric or GEJ adenocarcinoma whose tumors are CLDN18.2 positive (defined as ≥75% of tumor cells demonstrating moderate to strong membranous CLDN18 immunohistochemical staining) for treatment with VYLOY in combination with fluoropyrimidine- and platinum-containing chemotherapy using an FDA-approved test
Information on FDA-approved tests for the detection of CLDN18.2 is available at
The efficacy of VYLOY in combination with mFOLFOX6 was evaluated in SPOTLIGHT (NCT03504397), a double‑blind, randomized, multicenter study that enrolled 565 patients with locally advanced unresectable or metastatic HER2-negative gastric or GEJ adenocarcinoma whose tumors were CLDN18.2 positive. CLDN18.2 positivity (defined as ≥75% of tumor cells demonstrating moderate to strong membranous CLDN18 staining) was determined by immunohistochemistry on gastric or GEJ tumor tissue specimens from all patients with the VENTANA CLDN18 (43‑14A) RxDx Assay performed in a central laboratory. Patients were excluded from the study if they had a complete or partial gastric outlet syndrome, or history of central nervous system metastases.
Patients were randomized 1:1 to receive VYLOY in combination with mFOLFOX6 (n=283) or placebo in combination with mFOLFOX6 (n=282). VYLOY was administered intravenously at an initial dose of 800 mg/m2(Day 1 of cycle 1) followed by subsequent doses of 600 mg/m2every 3 weeks in combination with up to 12 treatments (4 cycles) of mFOLFOX6 (oxaliplatin 85 mg/m2, folinic acid (leucovorin or local equivalent) 400 mg/m2, fluorouracil 400 mg/m2given as a bolus and fluorouracil 2400 mg/m2given as a continuous infusion) administered on Days 1, 15 and 29 of a 42-day cycle. After 12 treatments, patients were allowed to continue treatment with VYLOY, 5-fluorouracil and folinic acid (leucovorin or local equivalent) at the discretion of the investigator, until progression of disease or unacceptable toxicity.
Treatment with VYLOY continued until RECIST v1.1-defined progression of disease as determined by an independent review committee (IRC) or a subsequent anticancer treatment was initiated. Tumor assessments were performed every 9 weeks up to and including Week 54, then every 12 weeks thereafter.
The major efficacy outcome measure was progression free survival (PFS) as assessed per RECIST v1.1 by IRC. Additional efficacy outcome measures were overall survival (OS), objective response rate (ORR) and duration of response (DOR) as assessed per RECIST v1.1 by IRC.
The study population characteristics were median age of 61 (range: 20-86); 62% were male; 48% were White, 34% Asian, 3.0% American Indian or Alaska, 1.2% Black or African American, 4.1% other racial groups, and race in 9% was unknown or missing; 78% non-Hispanic or Latino, 13% Hispanic or Latino, and ethnicity in 10% was missing; 98% had ECOG performance status (PS) of 0 or 1; 76% had gastric cancer, 24% had GEJ cancer; 84% were metastatic, 16% were locally advanced; and 29% had undergone prior gastrectomy. Subsequent anticancer therapy was received by 135 (48%) patients in the VYLOY in combination with mFOLFOX6 arm and 148 (53%) patients in the placebo in combination with mFOLFOX6 arm.
VYLOY in combination with mFOLFOX6 demonstrated a statistically significant improvement in PFS and OS compared with placebo in combination with mFOLFOX6.
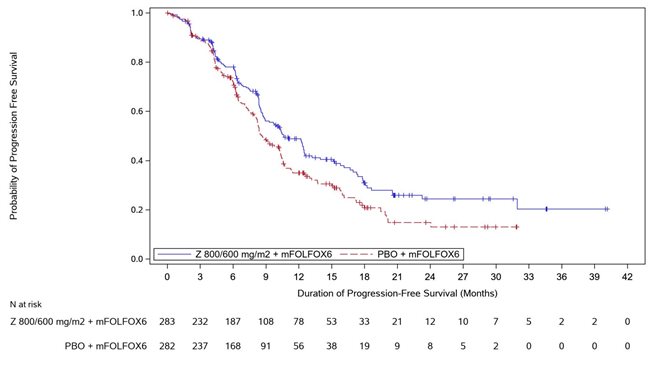
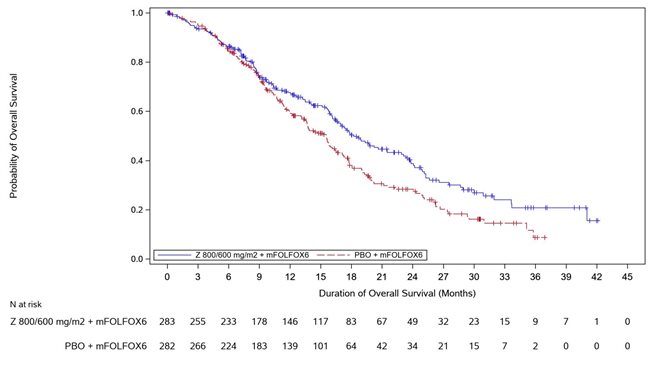
Table 7, Figures 1and 2summarize the efficacy results for the SPOTLIGHT study.
Endpoint | VYLOY with mFOLFOX6 n=283 | Placebo with mFOLFOX6 n=282 |
|---|---|---|
Progression Free Survival | ||
Number (%) of patients with events | 146 (51.6) | 167 (59.2) |
Median in months (95% CI)Based on Kaplan-Meier estimate. | 10.6 (8.9, 12.5) | 8.7 (8.2, 10.3) |
Hazard ratio (95% CI)Stratification factors were region, number of metastatic sites and prior gastrectomy from IRT. , Based on a stratified Cox proportional hazards model. | 0.751 (0.598, 0.942) | |
1-sided p-value , Based on a 1-sided stratified log-rank test. | 0.0066 | |
Overall survival | ||
Number (%) of patients with events | 149 (52.7) | 177 (62.8) |
Median in months (95% CI) | 18.2 (16.4, 22.9) | 15.5 (13.5, 16.5) |
Hazard ratio (95% CI) , | 0.750 (0.601, 0.936) | |
1-sided p-value , | 0.0053 | |
Objective Response Rate (CR + PR) Based on confirmed response. | ||
ORR (%) (95% CI)Based on binomial distribution (Clopper-Pearson). | 40.3 (34.5, 46.3) | 39.7 (34.0, 45.7) |
Complete response rate (%) | 14 (4.9) | 8 (2.8) |
Partial response rate (%) | 100 (35.3) | 104 (36.9) |
Duration of Response | N=114 | N=112 |
Median in months (95% CI) | 10.3 (8.3, 10.9) | 10.5 (7.7, 13.3) |
The efficacy of VYLOY in combination with CAPOX was evaluated in GLOW (NCT03653507), a double-blind, randomized, multicenter study that enrolled 507 patients with locally advanced unresectable or metastatic HER2-negative gastric or GEJ adenocarcinoma whose tumors were CLDN18.2 positive. CLDN18.2 positivity (defined as ≥75% of tumor cells demonstrating moderate to strong membranous CLDN18 staining) was determined by immunohistochemistry on gastric or GEJ tumor tissue specimens from all patients with the VENTANA CLDN18 (43-14A) RxDx Assay performed in a central laboratory. Patients were excluded from the study if they had a complete or partial gastric outlet syndrome, or history of central nervous system metastases.
Patients were randomized 1:1 to receive VYLOY in combination with CAPOX (n=254) or placebo in combination with CAPOX (n=253). VYLOY was administered intravenously at an initial dose of 800 mg/m2(Day 1 of cycle 1) followed by a subsequent dose of 600 mg/m2every 3 weeks in combination with up to 8 treatments (8 cycles) of CAPOX administered on Day 1 (oxaliplatin 130 mg/m2) and on Days 1 to 14 (capecitabine 1000 mg/m2) of a 21-day cycle. After 8 treatments of oxaliplatin, patients were allowed to continue treatment of VYLOY and capecitabine at the discretion of the investigator, until progression of disease or unacceptable toxicity.
Treatment with VYLOY continued until RECIST v1.1-defined progression of disease as determined by IRC or subsequent anticancer treatment was initiated. Tumor assessments were performed every 9 weeks up to and including Week 54, then every 12 weeks thereafter.
The major efficacy outcome measure was PFS as assessed per RECIST v1.1 by IRC. Additional efficacy outcome measures were OS, ORR, and DOR as assessed per RECIST v1.1 by IRC.
The study population characteristics were median age of 60 years (range: 21-83); 62% were male; 62% were Asian, 36% were White and race in 1.4% was missing; 95% non-Hispanic or Latino, 3.4% were Hispanic or Latino and ethnicity in 1.4% was missing; 99% had ECOG performance status (PS) of 0 or 1; 84% had primary gastric cancer, 16% had primary gastroesophageal adenocarcinoma; 88% were metastatic, 12% were locally advanced; and 27% had undergone prior gastrectomy.
VYLOY in combination with CAPOX demonstrated a statistically significant improvement in PFS and OS compared with placebo in combination with CAPOX.
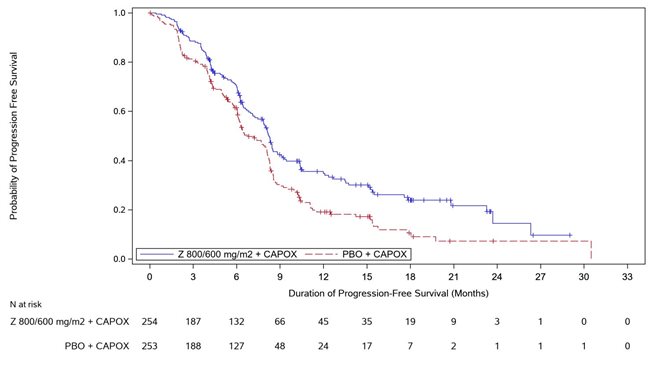
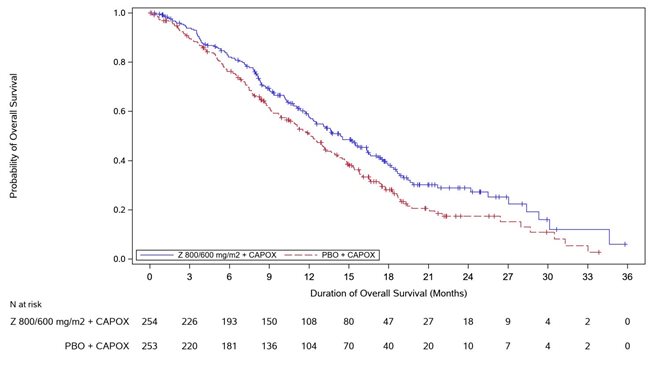
Table 8, Figures 3and 4summarize the efficacy results for the GLOW study.
Endpoint | VYLOY with CAPOX n=254 | Placebo with CAPOX n=253 |
|---|---|---|
Progression Free Survival | ||
Number (%) of patients with events | 137 (53.9) | 172 (68.0) |
Median in months (95% CI)Based on Kaplan-Meier estimate. | 8.2 (7.5, 8.8) | 6.8 (6.1, 8.1) |
Hazard ratio (95% CI)Stratification factors were region, number of metastatic sites and prior gastrectomy from IRT. , Based on a stratified Cox proportional hazards model. | 0.687 (0.544, 0.866) | |
1-sided p-value , Based on a 1-sided stratified log-rank test. | 0.0007 | |
Overall survival | ||
Number (%) of patients with events | 144 (56.7) | 174 (68.8) |
Median in months (95% CI) | 14.4 (12.3, 16.5) | 12.2 (10.3, 13.7) |
Hazard ratio (95% CI) , | 0.771 (0.615, 0.965) | |
1-sided p-value , | 0.0118 | |
Objective Response Rate (CR + PR) Based on confirmed response. | ||
ORR (%) (95% CI)Based on binomial distribution (Clopper-Pearson). | 32.3 (26.6, 38.4) | 31.2 (25.6, 37.3) |
Complete response rate (%) | 6 (2.4) | 2 (0.8) |
Partial response rate (%) | 76 (29.9) | 77 (30.4) |
Duration of Response | N=82 | N=79 |
Median in months (95% CI) | 8.3 (6.3, 11.4) | 6.2 (6.0, 7.6) |




• Administer by intravenous infusion only.Do not administer VYLOY as an intravenous push or bolus. ()2.6 Administration• Administer VYLOY as an intravenous infusion only. Do NOT administer as an intravenous push or bolus.• If VYLOY and fluoropyrimidine- and platinum-containing chemotherapy are administered on the same day, VYLOY must be administered first.• No incompatibilities have been observed witho closed system transfer devices composed of PP, PE, stainless steel, silicone (rubber/oil/resin), polyisoprene, PVC with TOTM plasticizer, acrylonitrile-butadiene-styrene (ABS) copolymer, methyl methacrylate-ABS copolymer, thermoplastic elastomer, polytetrafluoroethylene, polycarbonate, PES, acrylic copolymer, polybutylene terephthalate, PB, or EVA copolymer.o central ports composed of silicone rubber, titanium alloy or PVC with TOTM plasticizer.
• In-line filters (pore size of 0.2 μm composed of materials listed above) are recommended to be used during administration.• Do NOT co-administer other drugs through the same infusion line.• Immediately administer the infusion as described in Table 2. To minimize the risk of adverse reactions, begin each infusion at a slower rate for 30 to 60 minutes; if tolerated, gradually increase the rate as described in Table 2.• If the infusion time exceeds the recommended storage time (6 hours from end of preparation of infusion solution at room temperature or 16 hours from end of preparation of infusion solution under refrigeration), the infusion bag must be discarded and a new infusion bag prepared to continue the infusion.
Infusion Rate RecommendationsTable 2. Infusion Rates Recommended for Each VYLOY Infusion VYLOY DoseInitial Infusion Rate(first 30-60 minutes)In the absence of adverse reactions after 30 to 60 minutes, the infusion rate can be increased to the subsequent infusion rate as tolerated.Subsequent Infusion RateFirst Dose
800 mg/m2
100 mg/m2/hr
200-265 mg/m2/hr
Subsequent Doses
600 mg/m2every 3 weeks
75 mg/m2/hr
150-265 mg/m2/hr
or
or
or
400 mg/m2every 2 weeks
50 mg/m2/hr
100-200 mg/m2/hr
• The recommended first dose of VYLOY is 800 mg/m2 followed by 600 mg/m2 every 3 weeks or 400 mg/m2 every 2 weeks. ()2.3 Recommended DosageAdminister VYLOY in combination with fluoropyrimidine- and platinum-containing chemotherapy as follows:
• First dose: 800 mg/m2intravenously.• Subsequent doses:o 600 mg/m2intravenously every 3 weeks, oro 400 mg/m2intravenously every 2 weeks.
• Continue treatment until disease progression or unacceptable toxicity.
For injection: 100 mg and 300 mg of zolbetuximab-clzb as a white to off-white lyophilized powder in a single-dose vial.
Lactation: Advise not to breastfeed. (
There are no data on the presence of zolbetuximab-clzb in human milk, the effects on the breastfed child, or the effects on milk production. Because antibodies may be excreted in human milk and because of the potential for adverse reactions in a breastfed child, advise a lactating woman not to breastfeed during treatment with VYLOY and for 8 months after the last dose.
None.
• Hypersensitivity reactions including serious anaphylaxis reactions and serious and fatal infusion-related reactions have occurred. Monitor patients during and for at least 2 hours after infusion with VYLOY. Interrupt, slow the rate of infusion or permanently discontinue VYLOY based on severity and type of reaction. Premedicate with antihistamines for subsequent infusions after a hypersensitivity reaction. (,2.4 Dosage Modifications for Adverse ReactionsNo dose reduction for VYLOY is recommended. Adverse reactions for VYLOY are managed by reducing the infusion rate, interruption of the infusion, withholding the dose, and/or permanently discontinuing VYLOY as described in Table 1.
Table 1. Recommended Dose Modifications for VYLOY for Adverse Reactions Adverse ReactionSeverityToxicity was graded per National Cancer Institute Common Terminology Criteria for Adverse Events Version 5.0 (NCI-CTCAE v5.0).Dose ModificationHypersensitivity or Infusion-related reactions
[see Warnings and Precautions ].Grade 2
• Interrupt the infusion until Grade ≤1, then resume at a reduced infusion rate for the remaining infusion.• Premedicate and administer the next infusion per the infusion rates in Table 2.
Grade 3Follow Grade 2 management for Grade 3 infusion-related nausea and vomiting.or 4 or anaphylaxis
• Immediately stop the infusion and permanently discontinue.
)5.1 Hypersensitivity reactions, including anaphylaxis reactions, and infusion related reactionsHypersensitivity reactions, including serious anaphylaxis reactions, and serious and fatal infusion-related reactions (IRR) have been reported in clinical studies when VYLOY has been administered.
Any grade hypersensitivity reactions, including anaphylactic reactions, occurring with VYLOY in combination with mFOLFOX6 or CAPOX was 18%. Severe (Grade 3 or 4) hypersensitivity reactions, including anaphylactic reactions, occurred in 2% of patients. Seven patients (1.3%) permanently discontinued VYLOY for hypersensitivity reactions, including two patients (0.4%) who permanently discontinued VYLOY due to anaphylactic reactions. Seventeen (3.2%) patients required dose interruption, and three patients (0.6%) required infusion rate reduction due to hypersensitivity reactions.
All grade IRRs occurred in 3.2% in patients administered VYLOY in combination with mFOLFOX6 or CAPOX. Severe (Grade 3) IRRs occurred in 2 (0.4%) patients who received VYLOY. An IRR led to permanent discontinuation of VYLOY in 2 (0.4%) patients and dose interruption in 7 (1.3%) patients. The infusion rate was reduced for VYLOY for 2 (0.4%) patients due to an IRR.
Monitor patients during infusion with VYLOY and for 2 hours after completion of infusion or longer if clinically indicated, for hypersensitivity reactions with symptoms and signs that are highly suggestive of anaphylaxis (urticaria, repetitive cough, wheeze and throat tightness/change in voice). Monitor patients for signs and symptoms of IRRs including nausea, vomiting, abdominal pain, salivary hypersecretion, pyrexia, chest discomfort, chills, back pain, cough and hypertension.
If a severe or life-threatening hypersensitivity or IRR reaction occurs, discontinue VYLOY permanently, treat symptoms according to standard medical care, and monitor until symptoms resolve. For any Grade 2 hypersensitivity or IRR, interrupt the VYLOY infusion until Grade ≤1, then resume at a reduced infusion rate for the remaining infusion. Premedicate the patient with antihistamines for the subsequent infusions, administer per the infusion rates in Table 2and closely monitor the patient for symptoms and signs of a hypersensitivity reaction. The infusion rate may be gradually increased as tolerated
[see Dosage and Administration ].• Severe nausea and vomiting: Premedicate patients with antiemetics prior to each infusion. Interrupt or permanently discontinue VYLOY based on the severity of the nausea and/or vomiting. Manage patients during and after infusion with antiemetics or fluid replacement. (,2.4 Dosage Modifications for Adverse ReactionsNo dose reduction for VYLOY is recommended. Adverse reactions for VYLOY are managed by reducing the infusion rate, interruption of the infusion, withholding the dose, and/or permanently discontinuing VYLOY as described in Table 1.
Table 1. Recommended Dose Modifications for VYLOY for Adverse Reactions Adverse ReactionSeverityToxicity was graded per National Cancer Institute Common Terminology Criteria for Adverse Events Version 5.0 (NCI-CTCAE v5.0).Dose ModificationHypersensitivity or Infusion-related reactions
[see Warnings and Precautions ].Grade 2
• Interrupt the infusion until Grade ≤1, then resume at a reduced infusion rate for the remaining infusion.• Premedicate and administer the next infusion per the infusion rates in Table 2.
Grade 3Follow Grade 2 management for Grade 3 infusion-related nausea and vomiting.or 4 or anaphylaxis
• Immediately stop the infusion and permanently discontinue.
)5.2 Severe Nausea and VomitingVYLOY is emetogenic. Nausea and vomiting occurred more often during the first cycle of treatment.
All grade nausea and vomiting occurred in 82% and 67%, respectively, of patients treated with VYLOY in combination with mFOLFOX6 and 69% and 66% in combination with CAPOX, respectively. Severe (Grade 3) nausea occurred in 16% and 9% of patients treated with VYLOY in combination with mFOLFOX6 or CAPOX respectively. Severe (Grade 3) vomiting occurred in 16% and 12% of patients treated with VYLOY in combination with mFOLFOX6 or CAPOX.
Nausea led to permanent discontinuation of VYLOY in combination with mFOLFOX6 or CAPOX in 18 (3.4%) patients and dose interruption in 147 (28%) patients. Vomiting led to permanent discontinuation of VYLOY in combination with mFOLFOX6 or CAPOX in 20 (3.8%) patients and dose interruption in 150 (28%) patients.
Pretreat with antiemetics prior to each infusion of VYLOY
[see Dosage and Administration ]. Manage patients during and after infusion with antiemetics or fluid replacement.Interrupt the infusion, or permanently discontinue VYLOY based on severity
[see Dosage and Administration ].