Xhance
(Fluticasone Propionate)Xhance Prescribing Information
Indications and Usage (
XHANCE is a corticosteroid indicated for the treatment of:
• Chronic rhinosinusitis with nasal polyps (CRSwNP) in adults. ()1.1 Chronic Rhinosinusitis with Nasal PolypsXHANCE is indicated for the treatment of chronic rhinosinusitis with nasal polyps (CRSwNP) in adults.
• Chronic rhinosinusitis without nasal polyps (CRSsNP) in adults. ()1.2 Chronic Rhinosinusitis without Nasal PolypsXHANCE is indicated for the treatment of chronic rhinosinusitis without nasal polyps (CRSsNP) in adults.
• Recommended Dosage: 186 mcg (1 spray per nostril) or 372 mcg (2 sprays per nostril) twice daily. ()2.1 Recommended DosageThe recommended dosage of XHANCE is 186 mcg (1 spray per nostril) or 372 mcg (2 sprays per nostril) twice daily (total daily dose of 372 mcg or 744 mcg). The maximum total daily dosage should not exceed 2 sprays in each nostril twice daily (total daily dose of 744 mcg).
Patients should use XHANCE at regular intervals since its effectiveness depends on regular use. Individual patients will experience a variable time to onset and different degrees of symptom relief.
The safety and efficacy of XHANCE when administered in excess of recommended doses have not been established.
• For nasal use only. Shake before use. Prime before initial use. ()2.2 Administration Information• Shake XHANCE before each use.• Administer XHANCE by the nasal route only. Avoid spraying directly on the nasal septum.
PrimingBefore initial use, prime XHANCE by first gently shaking and then pressing the bottle 7 times or until a fine mist appears. Direct the spray into the air, away from the face. When XHANCE has not been used for ≥ 7 days, prime the pump again by shaking and releasing 2 sprays into the air, away from the face.
Administration InstructionsXHANCE is delivered into the nose by actuating the pump spray into one nostril while simultaneously blowing (exhaling) into the mouthpiece of the device. To administer XHANCE, insert the tapered tip of the cone-shaped nosepiece deep into one nostril and form a tight seal between the nosepiece and the nostril. Next, place the flexible mouthpiece into the mouth, bending it as necessary to maintain a tight seal with the nostril. Blow into the mouthpiece, and while continuing to blow, push the bottle up to actuate the spray pump. Continuing to blow through the mouth, but not inhaling or exhaling through the nose, at the time of actuation is important to achieve intended drug deposition. Repeat the process in the other nostril for a full dose
[see Instructions for Use].• XHANCE is delivered into the nose by actuating the pump spray into one nostril while simultaneously blowing into the mouthpiece of the device. ()2.2 Administration Information• Shake XHANCE before each use.• Administer XHANCE by the nasal route only. Avoid spraying directly on the nasal septum.
PrimingBefore initial use, prime XHANCE by first gently shaking and then pressing the bottle 7 times or until a fine mist appears. Direct the spray into the air, away from the face. When XHANCE has not been used for ≥ 7 days, prime the pump again by shaking and releasing 2 sprays into the air, away from the face.
Administration InstructionsXHANCE is delivered into the nose by actuating the pump spray into one nostril while simultaneously blowing (exhaling) into the mouthpiece of the device. To administer XHANCE, insert the tapered tip of the cone-shaped nosepiece deep into one nostril and form a tight seal between the nosepiece and the nostril. Next, place the flexible mouthpiece into the mouth, bending it as necessary to maintain a tight seal with the nostril. Blow into the mouthpiece, and while continuing to blow, push the bottle up to actuate the spray pump. Continuing to blow through the mouth, but not inhaling or exhaling through the nose, at the time of actuation is important to achieve intended drug deposition. Repeat the process in the other nostril for a full dose
[see Instructions for Use].
Nasal spray: Each spray delivers 93 mcg of fluticasone propionate. One unit provides 120 metered sprays.
Hepatic impairment: Monitor patients for signs of increased drug exposure. (
Formal pharmacokinetic trials using XHANCE have not been conducted in subjects with hepatic impairment. Since fluticasone propionate is predominantly cleared by hepatic metabolism, impairment of liver function may lead to accumulation of fluticasone propionate in plasma. Therefore, patients with hepatic disease should be closely monitored.
XHANCE is contraindicated in patients with hypersensitivity to any of the ingredients
Hypersensitivity reactions (e.g., anaphylaxis, angioedema, urticaria, contact dermatitis, rash, hypotension, and bronchospasm) have been reported after administration of fluticasone propionate. XHANCE is contraindicated in patients with known hypersensitivity to fluticasone propionate or any of the ingredients of XHANCE. Discontinue XHANCE if such reactions (e.g., anaphylaxis, angioedema, urticaria, contact dermatitis, rash, hypotension, and bronchospasm) occur
The active component of XHANCE is fluticasone propionate, a corticosteroid, having the chemical name
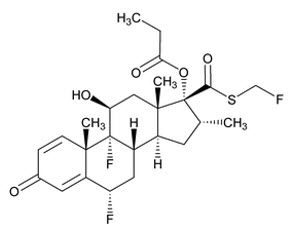
Fluticasone propionate is a white powder with a molecular weight of 500.57, and the empirical formula is C25H31F3O5S. It is practically insoluble in water, freely soluble in dimethylformamide, sparingly soluble in acetone and dichloromethane, and slightly soluble in 96% ethanol.
XHANCE (fluticasone propionate) nasal spray, 93 mcg, for nasal administration, with an Optinose exhalation delivery system that delivers an aqueous suspension of microfine fluticasone propionate having a particle size distribution in the range of 0 to 5 microns for topical nasal administration by means of a metering, atomizing spray pump and exhaled breath. XHANCE also contains microcrystalline cellulose and carboxymethylcellulose sodium, dextrose, benzalkonium chloride, polysorbate 80, edetate disodium dihydrate, sodium hydroxide and hydrochloric acid (to adjust pH), and purified water, and has a pH between 5 and 7.
Before initial use, prime XHANCE by gently shaking and then pressing the amber glass bottle 7 times or until a fine mist appears. Once primed, XHANCE contains 120 metered sprays. When XHANCE has not been used for ≥ 7 days, prime again by releasing 2 sprays into the air, away from the face
After priming, each spray delivers 93 mcg of fluticasone propionate in an aqueous suspension through the cone-shaped nosepiece. The system also has a flexible mouthpiece. Within the device is a non‑removable amber glass bottle with a metering spray pump, an applicator, and a valve that prevents release of breath until the bottle is pressed. A base covers the bottom of the bottle, and a removable orange cap covers both the nosepiece and mouthpiece.
