Get your patient on Xyosted - Testosterone Enanthate injection (Testosterone Enanthate)
Xyosted - Testosterone Enanthate injection prescribing information
INDICATIONS AND USAGE
XYOSTED (testosterone enanthate) injection is an androgen indicated for testosterone replacement therapy in adult males for conditions associated with a deficiency or absence of endogenous testosterone.
- Primary hypogonadism (congenital or acquired): testicular failure due to cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome, orchiectomy, Klinefelter's syndrome, chemotherapy, or toxic damage from alcohol or heavy metals. These men usually have low serum testosterone concentrations and gonadotropins (follicle-stimulating hormone [FSH], luteinizing hormone [LH]) above the normal range.
- Hypogonadotropic hypogonadism (congenital or acquired): gonadotropin or luteinizing hormone-releasing hormone (LHRH) deficiency or pituitary-hypothalamic injury from tumors, trauma, or radiation. These men have low testosterone serum concentrations but have gonadotropins in the normal or low range.
Limitations of Use:
- Safety and efficacy of XYOSTED in men with “age-related hypogonadism” has not been established.
- Safety and efficacy of XYOSTED in males less than 18 years old have not been established [see Use in Specific Populations (8.4 )] .
DOSAGE AND ADMINISTRATION
Confirmation of Hypogonadism Before Initiation of XYOSTED
Prior to initiating XYOSTED, confirm the diagnosis of hypogonadism by ensuring that serum testosterone concentrations have been measured in the morning on at least two separate days and that these serum testosterone concentrations are below the normal range.
Starting Dose and Dose Adjustment
The starting dose of XYOSTED is 75 mg, administered subcutaneously in the abdominal region once a week.
Dose adjustment
Measure total testosterone trough concentrations (measured 7 days after the most recent dose) following 6 weeks of dosing, following 6 weeks after dose adjustment, and periodically while on treatment with XYOSTED. A trough concentration between 350 ng/dL and 650 ng/dL generally provides testosterone exposures in the normal range during the entire dosing interval.
Decrease the dose by 25 mg if the total testosterone trough concentration (C trough ) is ≥650 ng/dL. Increase the dose by 25 mg if the total testosterone C trough is <350 ng/dL. Maintain the same dose if the total testosterone C trough is ≥350 ng/dL and <650 ng/dL.
Important Administration Instructions
XYOSTED is for subcutaneous injection in the abdominal region only. Avoid intramuscular or intravascular injection.
Instruct patients on the proper use of XYOSTED and direct them to use the proper injection site. Refer to the Instructions for Use for proper use of XYOSTED.
Visually inspect XYOSTED for particulate matter and discoloration prior to administration. Do not use if the liquid is cloudy or if visible particles are present.
Do not use XYOSTED if the seal is broken. Discard unused portion.
DOSAGE FORMS AND STRENGTHS
XYOSTED injection is available as 0.5 mL of a sterile, preservative-free and nonpyrogenic, clear, colorless to yellow solution containing testosterone enanthate. It is supplied in a single-dose syringe assembled in an autoinjector for subcutaneous administration. XYOSTED is available in three dosage strengths:
50 mg/0.5 mL
75 mg/0.5 mL
100 mg/0.5 mL
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
XYOSTED is contraindicated in pregnant women. Testosterone is teratogenic and may cause fetal harm when administered to a pregnant woman based on data from animal studies and its mechanism of action [see Contraindications (4 ) and Clinical Pharmacology (12.1 )] . Exposure of a female fetus to androgens may result in varying degrees of virilization. In animal developmental studies, exposure to testosterone in utero resulted in hormonal and behavioral changes in offspring and structural impairments of reproductive tissues in female and male offspring. These studies did not meet current standards for nonclinical development toxicity studies.
Data Animal Data
In developmental studies conducted in rats, rabbits, pigs, sheep and rhesus monkeys, pregnant animals received intramuscular injection of testosterone during the period of organogenesis. Testosterone treatment at doses that were comparable to those used for testosterone replacement therapy resulted in structural impairments in both female and male offspring. Structural impairments observed in females included increased ano-genital distance, phallus development, empty scrotum, no external vagina, intrauterine growth retardation, reduced ovarian reserve, and increased ovarian follicular recruitment. Structural impairments seen in male offspring included increased testicular weight, larger seminal tubular lumen diameter, and higher frequency of occluded tubule lumen. Increased pituitary weight was seen in both sexes.
Testosterone exposure in utero also resulted in hormonal and behavioral changes in offspring. Hypertension was observed in pregnant females and offspring in rats exposed to doses approximately twice those used for testosterone replacement therapy.
Lactation
Risk Summary
XYOSTED is not indicated for use in females.
Females and Males of Reproductive Potential
Infertility
During treatment with large doses of exogenous androgens, including XYOSTED, spermatogenesis may be suppressed through feedback inhibition of the hypothalamic-pituitary-testicular axis [ see Warnings and Precautions (5.8 ) ] . Reduced fertility is observed in some men taking testosterone replacement therapy. The impact on fertility may be irreversible.
Pediatric Use
Safety and effectiveness of XYOSTED in pediatric patients less than 18 years old have not been established. Improper use may result in acceleration of bone age and premature closure of epiphyses.
Geriatric Use
There have not been sufficient numbers of geriatric patients in controlled clinical studies with XYOSTED to determine whether efficacy or safety in those over 65 years of age differs from younger subjects. Of the 283 patients enrolled in the 6-month and one-year efficacy and safety clinical study utilizing XYOSTED, 49 (17%) were over 65 years of age. Additionally, there are insufficient long-term safety data in geriatric patients to assess the potentially increased risk of cardiovascular disease and prostate cancer.
Geriatric patients treated with androgens may also be at risk for worsening of signs and symptoms of BPH [see Warnings and Precautions (5.4 )].
CONTRAINDICATIONS
XYOSTED is contraindicated in:
- Men with carcinoma of the breast or known or suspected carcinoma of the prostate [see Warnings and Precautions (5.4 )] .
- Women who are pregnant. Testosterone can cause virilization of the female fetus when administered to a pregnant woman [see Use in Specific Populations (8.1 )] .
- Men with known hypersensitivity to XYOSTED or any of its ingredients (testosterone enanthate and sesame oil).
WARNINGS AND PRECAUTIONS
Polycythemia
Increases in hematocrit reflective of increases in red blood cell mass may require discontinuation of XYOSTED.
Check that hematocrit is not elevated prior to initiating XYOSTED. Evaluate hematocrit approximately every 3 months while the patient is on XYOSTED. If hematocrit becomes elevated, stop XYOSTED until the hematocrit decreases to an acceptable level. If XYOSTED is restarted and again causes hematocrit to become elevated, stop XYOSTED permanently. An increase in red blood cell mass may increase the risk of thromboembolic events.
Venous Thromboembolism
There have been postmarketing reports of venous thromboembolic events, including deep vein thrombosis (DVT) and pulmonary embolism (PE), in patients using testosterone products, such as XYOSTED.
In the Testosterone Replacement therapy for Assessment of long-term Vascular Events and efficacy Response in hypogonadal men (TRAVERSE) Study, a randomized, double-blind, placebo-controlled, cardiovascular (CV) outcomes study, compared to placebo, topical testosterone gel was associated with a numerically higher incidence of VTE (1.7% vs 1.2%) which included DVT (0.6% vs 0.5%) and PE events (0.9% vs 0.5%) [see Adverse Reactions (6.1 )] .
Evaluate patients who report symptoms of pain, edema, warmth and erythema in the lower extremity for DVT and those who present with acute shortness of breath for PE. If a venous thromboembolic event is suspected, discontinue treatment with XYOSTED and initiate appropriate workup and management.
Worsening of Benign Prostatic Hyperplasia (BPH) and Potential Risk of Prostate Cancer
Patients with BPH treated with androgens are at an increased risk of worsening of signs and symptoms of BPH. Monitor patients with BPH for worsening signs and symptoms.
Patients treated with androgens may be at an increased risk for prostate cancer. Evaluate patients for prostate cancer prior to initiating and during treatment with androgens [see Contraindications (4 )] .
Blood Pressure Increases
XYOSTED can increase blood pressure. Ambulatory blood pressure monitoring (ABPM) demonstrated XYOSTED increased systolic/diastolic BP by an average of 3.9/1.5 mm Hg from baseline after 12 weeks of treatment in clinical trials [see Adverse Reactions (6.1 )]. In patients with hypertension on antihypertensive therapy, XYOSTED increased the mean systolic/diastolic BP by 3.9/1.3 mm Hg from baseline.
The CV risk associated with topical testosterone gel was evaluated in TRAVERSE, a randomized, double-blind, placebo-controlled, CV outcomes study in men with a history of CV disease or multiple CV risk factors. In TRAVERSE, topical testosterone gel increased mean systolic blood pressure by 1.0 mm Hg from baseline to 36 months, whereas a mean decrease from baseline of 0.5 mm Hg was observed in the placebo group at this timepoint, for a mean between-group difference of 1.5 mm Hg. However, the incidences of major adverse cardiovascular events (MACE), including cardiovascular death, nonfatal myocardial infarction [MI] and non-fatal stroke, were similar between treatment groups (7% for topical testosterone gel vs 7.3% for placebo) [See Adverse Reactions (6.1 )] .
Monitor blood pressure periodically in men using XYOSTED, especially men with hypertension. XYOSTED is not recommended for use in patients with uncontrolled hypertension.
Abuse of Testosterone and Monitoring of Serum Testosterone Concentrations
Testosterone has been subject to abuse, typically at doses higher than recommended for the approved indication and in combination with other anabolic androgenic steroids. Anabolic androgenic steroid abuse can lead to serious cardiovascular and psychiatric adverse reactions [see Drug Abuse and Dependence (9 )] .
If testosterone abuse is suspected, check serum testosterone concentrations to ensure they are within therapeutic range. However, testosterone levels may be in the normal or subnormal range in men abusing synthetic testosterone derivatives. Counsel patients concerning the serious adverse reactions associated with abuse of testosterone and anabolic androgenic steroids. Conversely, consider the possibility of testosterone and anabolic androgenic steroid abuse in suspected patients who present with serious cardiovascular or psychiatric adverse events.
Not for Use in Women
Potential for Adverse Effects on Spermatogenesis
With large doses of exogenous androgens, including XYOSTED, spermatogenesis may be suppressed through feedback inhibition of pituitary follicle-stimulating hormone (FSH) which could possibly lead to adverse effects on semen parameters including sperm count [see Use in Specific Populations (8.3 )] . Patients should be informed of this possible risk when deciding whether to use or to continue to use XYOSTED.
Hepatic Adverse Effects
Prolonged use of high doses of orally active 17-alpha-alkyl androgens (e.g., methyltestosterone) has been associated with serious hepatic adverse effects (peliosis hepatis, hepatic neoplasms, cholestatic hepatitis, and jaundice). Peliosis hepatis can be a life-threatening or fatal complication. Long-term therapy with intramuscular testosterone enanthate, which elevates blood levels for prolonged periods, has produced multiple hepatic adenomas. XYOSTED is not known to produce these adverse effects. Nonetheless, patients should be instructed to report any signs or symptoms of hepatic dysfunction (e.g., jaundice). If these occur, promptly discontinue XYOSTED while the cause is evaluated.
Edema
Androgens, including XYOSTED, may promote retention of sodium and water. Edema with or without congestive heart failure may be a serious complication in patients with preexisting cardiac, renal, or hepatic disease. In addition to discontinuation of the drug, diuretic therapy may be required.
Gynecomastia
Gynecomastia may develop and may persist in patients being treated for hypogonadism.
Sleep Apnea
Treatment with testosterone products including XYOSTED may potentiate sleep apnea in some patients, especially those with risk factors such as obesity or chronic lung disease.
Lipid Changes
Changes in the serum lipid profile may require dose adjustment of lipid lowering drugs or discontinuation of testosterone therapy. Monitor the lipid profile periodically, particularly after starting testosterone therapy.
Hypercalcemia
Androgens, including XYOSTED, should be used with caution in cancer patients at risk of hypercalcemia (and associated hypercalciuria). Monitor serum calcium concentrations regularly during treatment with XYOSTED in these patients.
Decreased Thyroxine-binding Globulin
Androgens, including XYOSTED, may decrease concentrations of thyroxine-binding globulin, resulting in decreased total T4 serum concentrations and increased resin uptake of T3 and T4. Free thyroid hormone concentrations remain unchanged, however, and there is no clinical evidence of thyroid dysfunction.
Risk of Depression and Suicide
Depression and suicidal ideation and behavior, including completed suicide, have occurred during clinical trials in patients treated with XYOSTED. Advise patients and caregivers to seek medical attention for manifestations of suicidal ideation or behavior, new onset or worsening depression, anxiety, or other mood changes.
ADVERSE REACTIONS
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of XYOSTED was evaluated in 2 clinical studies in a total of 283 men who received weekly subcutaneous doses for up to one year. All patients were started on 75 mg weekly, then the dose was titrated to 50 mg or 100 mg weekly, as needed, to achieve pre-dose total testosterone concentrations of ≥350 ng/dL and <650 ng/dL.
Table 1 summarizes adverse reactions (≥2%) reported in a one-year study with XYOSTED.
Table 1. Number (%) of Patients with Adverse Reactions ≥2% in a 1 Year study with XYOSTED
Preferred Term | Overall (N=150) n (%) |
Hematocrit increased | 21 (14.0) |
Hypertension | 19 (12.7) |
Prostatic specific antigen (PSA) increased | 18 (12.0) |
Injection site bruising | 10 (6.7) |
Headache | 8 (5.3) |
Back pain | 5 (3.3) |
Blood creatine phosphokinase increased | 5 (3.3) |
Injection site hemorrhage | 5 (3.3) |
Acne | 4 (2.7) |
Blood testosterone increased | 4 (2.7) |
Cough | 4 (2.7) |
Edema peripheral | 4 (2.7) |
Injection site erythema | 4 (2.7) |
Prostatitis | 4 (2.7) |
Urinary tract infection | 4 (2.7) |
Abdominal pain | 3 (2.0) |
Arthralgia | 3 (2.0) |
Fatigue | 3 (2.0) |
Hematuria | 3 (2.0) |
Polycythemia | 3 (2.0) |
Sleep apnea syndrome | 3 (2.0) |
Note: Includes events that started on or after the first dosing, or existed prior to the first dose and worsened in severity or relatedness after dosing. Percentage was calculated using the number of patients in the column heading as the denominator. | |
Table 2 summarizes the adverse reactions (≥2%) reported in a 6-month study with XYOSTED.
Table 2. Number (%) of Patients with Adverse Reactions ≥2% in a 6-Month Study with XYOSTED
Preferred Term | Overall (N = 133) n (%) |
Hematocrit increased | 11 (8.3) |
Injection site hemorrhage | 8 (6.0) |
Blood creatine phosphokinase increased | 5 (3.8) |
Injection site bruising | 5 (3.8) |
Prostatitis | 4 (3.0) |
Prostatic specific antigen (PSA) increased | 4 (3.0) |
Urinary tract infection | 4 (3.0) |
Fatigue | 3 (2.3) |
Hypertension | 3 (2.3) |
Insomnia | 3 (2.3) |
Nausea | 3 (2.3) |
Note: Includes events that started on or after the first dosing, or existed prior to the first dose and worsened in severity or relatedness after dosing. Percentage was calculated using the number of patients in the column heading as the denominator. | |
BP Increases in the 6-Month Clinical Study
In the 6-month clinical study, 24-hour ambulatory blood pressure monitoring (ABPM) was conducted in 133 patients, 113 of whom completed the study. ABPM was conducted at 3 distinct 24-hour time periods: at Baseline and following 6 and 12 weeks of XYOSTED therapy. A total of 62 patients had acceptable ABPM recordings at baseline and Week 12. In that group, the mean change in systolic and diastolic BP from baseline to Week 12 was + 3.9 mm Hg (95% CI 1.4-6.4) and + 1.5 mm Hg (95% CI 0.4-2.6), respectively. In patients with a history of hypertension who were receiving antihypertensive therapy, the mean ABPM systolic and diastolic BP increased by +3.9 mm Hg [95% CI 0.19-7.7] and +1.3 mm Hg [95% CI -0.37-3.0], respectively [n=33]). In patients with no history of hypertension, the mean ABPM systolic and diastolic blood pressure increased by +4.1 mm Hg [95% CI 0.43-7.8] and +1.9 mm Hg [95% CI 0.055-3.7], respectively [n=28]).
10% of XYOSTED-treated patients were either started on antihypertensive medications or required changes to their antihypertensive medication regimen during the 1-year study.
A total of 3 patients were reported to have an adverse reaction of hypertension (2 patients with hypertension and 1 patient with worsening hypertension), and 1 was reported to have an adverse reaction of increased blood pressure.
Increases in Hematocrit
Increases in hematocrit to ≥55% were reported for 12 of the 283 patients in the 2 clinical studies, representing 4.2% of patients who received XYOSTED for up to one year. While the studies did not pre-define clinical adverse events related to increased hematocrit, polycythemia was reported as a medically significant adverse event in the investigator’s clinical judgment in 1.8% of treated patients. XYOSTED dosing resulted in mean hemoglobin increases of 1.0 ± 1.1 g/dL at 6 months and 1.1 ± 1.4 g/dL at 1 year and mean hematocrit increases of 3.8 ± 3.4% at 6 months and 5.4 ± 3.4% at 1 year.
Injection Site Reactions
Injection site reactions, including injection site bruising, injection site hemorrhage, injection site erythema and injection site induration were reported in 36 of the 283 patients in the 2 clinical studies, representing 12.7% of patients who received XYOSTED for up to one year. No patients discontinued XYOSTED because of injection site reactions.
Depression and Suicide Attempts
Depression requiring discontinuation was reported in 2 of the 283 patients in the two clinical studies and suicide attempts (one complete and one incomplete) were reported in 2 additional patients, comprising a total of 4 patients, representing 1.4% of patients who received XYOSTED for up to one year.
Increases in Serum PSA
Increases in serum PSA concentrations, defined as an increase from baseline of at least 1.4 ng/mL, or PSA greater than 4 ng/mL, led to discontinuation in 4.6% of the 283 patients in the 2 clinical studies.
Cardiovascular Outcomes
TRAVERSE was a randomized, double-blind, cardiovascular outcomes study to assess the cardiovascular (CV) safety of topical testosterone gel compared to placebo in 5198 hypogonadal men aged 45 to 80 years with a history of CV disease or with multiple CV risk factors. The primary outcome was the incidence of the composite endpoint of major adverse cardiovascular events (MACE), consisting of CV death, non-fatal myocardial infarction (MI), and non-fatal stroke.
The mean duration of therapy was approximately 22 months. The mean duration of follow-up was 33 months. Approximately 61% of all patients discontinued topical testosterone gel or placebo therapy.
The mean patient age (±SD) was 63.3 (7.9) years, with 2452 patients aged 65 years or more (47%); 2847 (about 55%) patients had pre-existing cardiovascular disease, whereas 2357 patients (about 45%) had an elevated cardiovascular risk at baseline, and mean BMI was 35 kg/m 2 . Approximately 80% of patients were White, 17% were Black, and 3% were of other races or ethnic groups. Approximately 69%, 84%, and 93% had diabetes mellitus, hyperlipidemia, and hypertension, respectively.
The mean serum testosterone concentration at baseline in patients receiving topical testosterone gel was 220.4 ng/dL (n=2596). The mean serum testosterone concentrations at 12 months, 24 months, 36 months, and 48 months in patients receiving topical testosterone gel were 440.5 ng/dL (n=1683), 420.9 ng/dl (n=1125), 428.7 ng/dL (n=731), and 365.2 ng/dL (n=220), respectively.
For patients treated with topical testosterone gel, the incidence of MACE was 7.0% (n=182 events) and for those receiving placebo, the incidence of MACE was 7.3% (n=190 events). The study demonstrated noninferiority of topical testosterone gel versus placebo because the upper bound of 95% CI was less than the prespecified risk margin, of 1.5 for MACE (Hazard Ratio 0.96 [95% CI: 0.78, 1.17]).
Additional Adverse Reactions Reported in TRAVERSE
Additional adverse reactions reported in TRAVERSE at an incidence rate >2% in either treatment group and greater in topical testosterone gel versus placebo included: nonfatal arrythmias warranting intervention (5.2% vs 3.3%), atrial fibrillation (3.5% vs 2.4%), acute kidney injury (2.3% vs 1.5%) and bone fracture (3.5% vs 2.5%). For the adverse reaction of bone fracture, each event was adjudicated by clinical review.
DRUG INTERACTIONS
Insulin
Changes in insulin sensitivity or glycemic control may occur in patients treated with androgens. In diabetic patients, the metabolic effects of androgens may decrease blood glucose and, therefore, may necessitate a decrease in the dose of anti-diabetic medication.
Oral Anticoagulants
Changes in anticoagulant activity may be seen with androgens, therefore more frequent monitoring of international normalized ratio (INR) and prothrombin time are recommended in patients taking warfarin, especially at the initiation and termination of androgen therapy.
Corticosteroids
The concurrent use of testosterone with corticosteroids may result in increased fluid retention and requires careful monitoring, particularly in patients with cardiac, renal or hepatic disease.
Medications that May Also Increase Blood Pressure
Some prescription medications and nonprescription analgesic and cold medications contain drugs known to increase blood pressure. Concomitant administration of these medications with XYOSTED may lead to additional increases in blood pressure.
DESCRIPTION
XYOSTED (testosterone enanthate) injection contains testosterone enanthate, an ester derivative of an endogenous androgen, testosterone. Testosterone enanthate is a white to creamy white crystalline powder described by the chemical name (17β)-17-[(1-Oxoheptyl) oxy]-androst-4-en-3-one. Testosterone enanthate has the molecular formula C 26 H 40 O 3 , the molecular weight 400.59, and the molecular structure with the chemical formula:
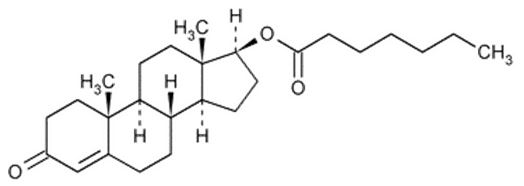
XYOSTED (testosterone enanthate) injection is a sterile, preservative-free, and nonpyrogenic colorless to pale yellow solution supplied in a single-dose syringe assembled in a pressure-assisted autoinjector for subcutaneous administration. Each XYOSTED autoinjector contains 50 mg, 75 mg, or 100 mg of testosterone in sesame oil to form 0.5 mL solution providing three product strengths of 50 mg/0.5 mL, 75 mg/0.5 mL, and 100 mg/0.5 mL.
CLINICAL PHARMACOLOGY
Mechanism of Action
Endogenous androgens, including testosterone and dihydrotestosterone (DHT) are responsible for the normal growth and development of the male sex organs and for maintenance of secondary sex characteristics. These effects include the growth and maturation of the prostate, seminal vesicles, penis, and scrotum; the development of male hair distribution, such as facial, pubic, chest, and axillary hair; laryngeal enlargement, vocal cord thickening, and alterations in body musculature and fat distribution.
Male hypogonadism, a clinical syndrome resulting from insufficient secretion of testosterone, has two main etiologies. Primary hypogonadism is caused by defects of the gonads, such as Klinefelter’s syndrome or Leydig cell aplasia, whereas secondary hypogonadism is the failure of the hypothalamus (or pituitary) to produce sufficient gonadotropins (FSH, LH).
Pharmacodynamics
No specific pharmacodynamic studies were conducted using XYOSTED.
Pharmacokinetics
Absorption and Bioavailability
XYOSTED delivers physiologic amounts of testosterone, producing circulating testosterone concentrations that approximate normal concentrations (300-1100 ng/dL) seen in healthy men.
Following weekly subcutaneous injection of XYOSTED for 12 weeks, serum testosterone concentrations reached a maximum after a median of 11.9 hours post-dose (range: 5.8-168.7 hours) then slowly declined (Figure 1). In this study, the starting dose of XYOSTED was 75 mg weekly then the XYOSTED dose was adjusted based upon total testosterone trough concentrations obtained following 6 weeks of dosing [see Dosage and Administration (2.2 )] . Steady state serum testosterone concentration was achieved by Week 6.
Figure 1. Mean (±SD) of Total Testosterone (TT) Concentration (ng/dL) Following Weekly Administration of XYOSTED for 12 Weeks (N=137)
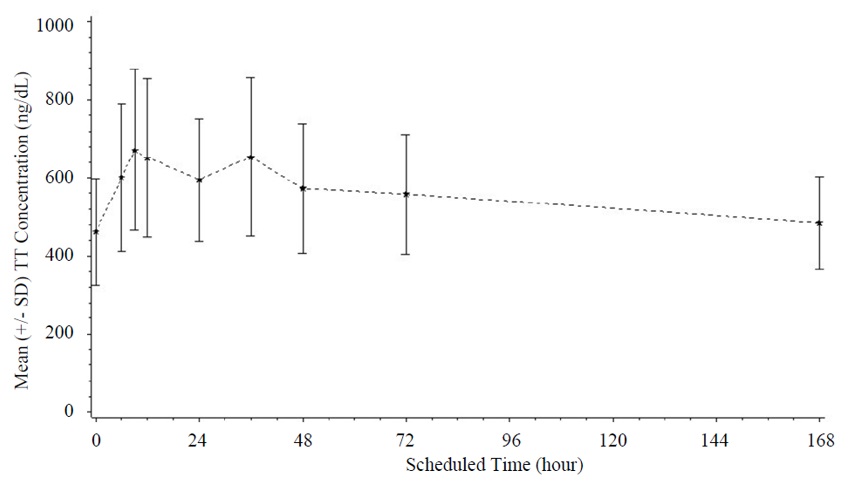
At Week 12, total testosterone concentrations were measured before and at specified times up to 168 hours after XYOSTED administration then analyzed for pharmacokinetic (PK) parameters (Table 3).
Table 3. Arithmetic Mean (SD) and Range for Total Testosterone Pharmacokinetic Parameters at Week 12 Following Weekly Administration of 50 mg, 75 mg, or 100 mg XYOSTED (N=137)
C avg (0-168 hr) (ng/dL) | C max (ng/dL) | T max (hr) a | C min (ng/dL) | AUC (0-168 hr) (ng·hr/dL) | |
Mean (SD) | 553 (127) | 790 (215) | 11.9 | 436 (109) | 92,955 (21,385) |
Min - Max | 276 - 1036 | 389 - 1410 | 6 - 168 | 166 - 788 | 46432 - 173,987 |
a Reported as median
Distribution
Circulating testosterone is primarily bound in serum to sex hormone-binding globulin (SHBG) and albumin. Approximately 40% of testosterone in plasma is bound to SHBG, 2% remains unbound (free), and the rest is loosely bound to albumin and other proteins.
Elimination
Metabolism
Testosterone enanthate is metabolized to testosterone via ester cleavage of the enanthate group. The mean (SD) maximum concentration of testosterone enanthate was 169 (68) ng/dL at Week 12 following weekly administration of XYOSTED.
Testosterone is metabolized to various 17-keto steroids through two different pathways. The major active metabolites of testosterone are estradiol and dihydrotestosterone (DHT). At pre-dose of Week 12 treatment, the mean DHT/testosterone ratio was 0.07 which was within the normal range.
Excretion
About 90% of a testosterone dose given intramuscularly is excreted in the urine as glucuronic and sulfuric acid-conjugates of testosterone or as metabolites. About 6% of a dose is excreted in the feces, mostly in the unconjugated form. Inactivation of testosterone occurs primarily in the liver.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
Testosterone has been tested by subcutaneous injection and implantation in mice and rats. In mice, the implant induced cervical-uterine tumors, which metastasized in some cases. There is suggestive evidence that injection of testosterone into some strains of female mice increases their susceptibility to hepatoma. Testosterone is also known to increase the number of tumors and decrease the degree of differentiation of chemically induced carcinomas of the liver in rats.
Mutagenicity
Testosterone was negative in the in vitro Ames and in the in vivo mouse micronucleus assays.
Impairment of Fertility
The administration of exogenous testosterone suppresses spermatogenesis in the rat, dog and non-human primates, which was reversible on cessation of the treatment.
CLINICAL STUDIES
XYOSTED was evaluated in a 52-week, open-label study (NCT02159469) to evaluate its efficacy and safety when administered subcutaneously once weekly to 150 adult males with hypogonadism. The study included a Screening Phase, a Treatment Titration Phase, and an Extended Treatment Phase.
Patients were trained on proper use of XYOSTED to self-administer the initial dose of 75 mg once weekly on the same day of the week and at approximately the same time (7:00 am ± 2 hours). The dose was increased by 25 mg at Week 7 if the Week 6 serum total testosterone concentration at the end of the dosing interval (C trough ) was <350 ng/dL, and was decreased by 25 mg if the C trough was ≥650 ng/dL.
The primary endpoint was the percentage of patients with a time-averaged serum total testosterone concentration (C avg ) over the 7-day dosing interval (0 to 168 hours) within the normal range (300 to 1100 ng/dL) at Week 12.
Secondary endpoints were the percentage of patients with a maximum total testosterone concentration (Cmax) above three predetermined limits: greater than 1500 ng/dL, between 1800 and 2500 ng/dL, and greater than 2500 ng/dL.
One hundred and thirty five (90%) of the 150 hypogonadal men who received XYOSTED had a serum total testosterone concentration C avg(0-168h) within the normal range (300 to 1100 ng/dL) at Week 12. There were no patients (0%) with C max >1500 ng/dL at Week 12.
HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
XYOSTED (testosterone enanthate) injection is provided as 0.5 mL of a sterile, preservative-free, and nonpyrogenic colorless to pale yellow solution in a single-dose syringe pre-assembled in an autoinjector for a single subcutaneous administration. XYOSTED is packaged in cartons containing four (4) or one (1) autoinjectors. XYOSTED is available in the following strengths and configurations.
- XYOSTED (testosterone enanthate) injection, USP, 50 mg/0.5 mL
- Carton of 4 autoinjectors NDC 54436-250-04
- Autoinjector NDC 54436-250-02
- XYOSTED (testosterone enanthate) injection, USP, 75 mg/0.5 mL
- Carton of 4 autoinjectors NDC 54436-275-04
- Carton of 1 autoinjector NDC 54436-275-05
- Autoinjector NDC 54436-275-02
- XYOSTED (testosterone enanthate) injection, USP, 100 mg/0.5 mL
- Carton of 4 autoinjectors NDC 54436-200-04
- Carton of 1 autoinjector NDC 54436-200-05
- Autoinjector NDC 54436-200-02
Before use, each autoinjector should be visually inspected. XYOSTED should be clear to light yellow in color and free of visible particles. Do not use if the liquid is cloudy or if visible particles are present. You may notice an air bubble, this is normal.
Storage and Handling
Do Not refrigerate or freeze. Use at room temperature.
- Store at controlled room temperature, 20°C to 25°C (68°F - 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F).
- Protect from light (keep in carton until time of use).
Instructions for Use
XYOSTED ® Testosterone Enanthate Injection USP CIII
Single-dose auto-injector. For a single subcutaneous administration. Administer one device weekly.
Read this Instructions for Use before you start using XYOSTED (ZYE-oh-sted) and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your healthcare provider about your medical condition or treatment.
Important Information about XYOSTED:
- Use XYOSTED exactly as your healthcare provider tells you to take it.
- Inject XYOSTED only 1 time each week. Do not use XYOSTED every day.
- Your healthcare provider will show you or your caregiver how to inject XYOSTED. You should not inject XYOSTED until you have been trained on the proper way to use it.
- Check XYOSTED before you inject it. XYOSTED should be clear to light yellow in color and should be free of visible particles.
- Do not use if the liquid is cloudy or if visible particles are present. You may see air bubble(s), this is normal.
- Do not use XYOSTED if the safety seal is broken or if the Auto-Injector appears broken, damaged or changed in any way.
- XYOSTED should be injected in the stomach (abdomen) area after cleaning the skin.
- Do not inject XYOSTED within 2 inches of the belly button (navel).
- Do not inject XYOSTED in any other areas of the body.
- Do not inject XYOSTED in areas where the skin is tender, bruised, red, scaly, hard, or has scars or stretch marks.
- Discard unused portion.
If you are not sure if XYOSTED was injected, or if you have a hard time giving the injection, do not inject another dose. Call your pharmacist or healthcare provider right away.
If you need help or instructions, call: 1-844-996-7833 |
|
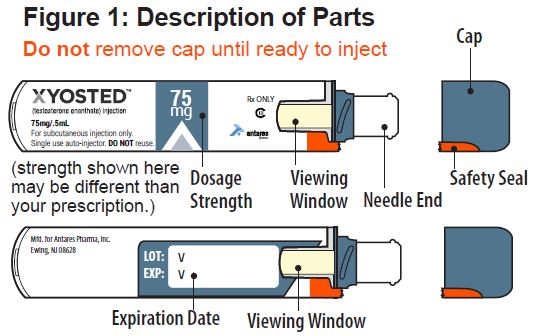
Supplies You Will Need
- 1 XYOSTED Auto-Injector
- 1 alcohol swab
- 1 cotton ball or gauze
Storage Conditions
- Do not refrigerate or freeze.
- Protect from light.
- Use at room temperature.
- Store at 68° - 77° F (20° - 25° C).
- Keep XYOSTED and all medicines away from children.
Inspect Auto-Injector
- Do not remove Cap until ready to inject.
- Inspect Auto-Injector for any visible damage. Do not use if it appears damaged or broken.
- Check the expiration date. Do not use if the expiration date has passed.
- Inspect the medicine through the Viewing Window; it should be clear to light yellow and free of visible particles ( see Figure 2 ).
Do not use if the medicine is cloudy or if visible particles are present. You may notice an air bubble, this is normal.
Select & Prep Injection Site
Wash your hands with soap and water. Wipe the abdomen injection site with an alcohol swab.
Allow the site to dry on its own. Do not fan or blow on the injection site. Do not touch the site again before injecting.
Only use the left or right side of the abdomen (belly) for injection sites. Do not use the area within 2 inches around your navel (belly button). See Figure 3.
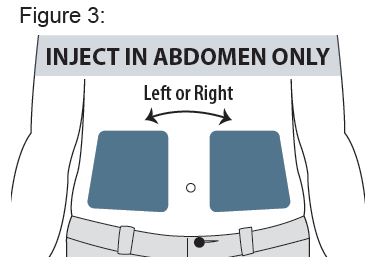
Do not use in areas where the skin is tender, bruised, red, scaly, or hard. Avoid areas with scars, tattoos, or stretch marks.
Administering Injection
1. Remove Cap
Twist the Cap to remove (this will also break the red safety seal). See Figure 4.
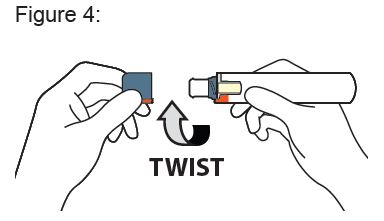
After the Cap is removed, a few drops of liquid may appear, this is normal. The Auto-Injector should be used or discarded after the Cap is removed. Do not re-Cap for later use.
 | Do not touch the Needle End of the Auto-Injector with your hand or fingers after the Cap is removed, doing so can cause injection and injury to your hands. |
2. Position Auto-Injector
Gently squeeze the abdomen injection site to create a raised area and hold that area firmly until after the injection is complete ( see Figure 5 ).
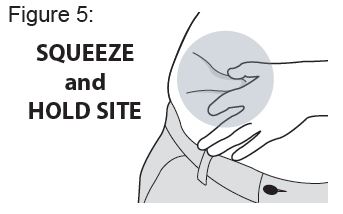
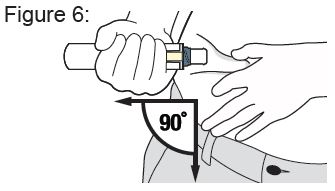
Place the Needle End of the Auto-Injector on the abdomen injection site.
Keep the Auto-Injector straight at a 90 degree angle to the abdomen injection site. ( see Figure 6 ).
3. Inject & Hold Down
Firmly push the Auto-Injector down on the abdomen site and continue to hold down after you hear the “CLICK” ( see Figure 7 ).
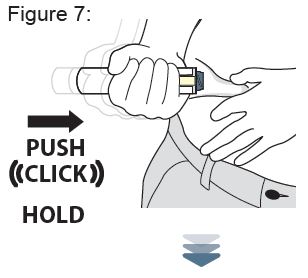
While holding the XYOSTED Auto-Injector down, slowly count from 1 to 10 to allow all of the medicine to be delivered ( see Figure 8 ).
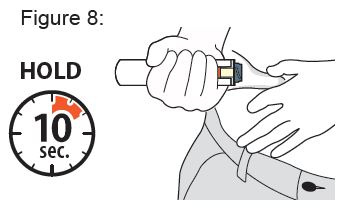
Keep holding the XYOSTED Auto-Injector down for a total of 10 seconds even if your injection is complete and the viewing window turns orange sooner.
It is normal if there is slight bleeding from the site after injection. If this occurs, hold a cotton ball or gauze on the area for a few seconds. Do not rub the area.
4. Inspect Viewing Window
After injecting, inspect the Viewing Window. It should be orange, confirming the dose was administered ( see Figure 9 ).
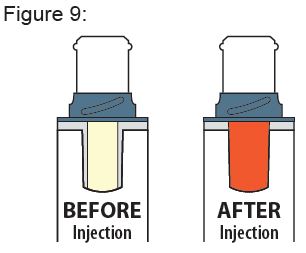
If the Viewing Window is not orange:
- Do not use another Auto-Injector.
- Do not attempt another injection.
- Call your healthcare provider or 1-844-996-7833 for assistance.
Disposal After Injection
 | After completing your injection, dispose of the XYOSTED Auto-Injector and Cap in an FDA-cleared sharps disposal container immediately after use. |
Do not dispose of the XYOSTED Auto-Injector in your household trash.
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- upright and stable during use
- leak-resistant
- properly labeled to warn of hazardous waste inside the container
When your sharps disposal container is almost full, you will need to follow your community guidelines for the proper way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal .
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
This Instructions for Use has been approved by the U.S. Food and Drug Administration
LB-0118 V08
Manufactured for: Antares Pharma, Inc. Ewing, NJ 08628 03/2025
Mechanism of Action
Endogenous androgens, including testosterone and dihydrotestosterone (DHT) are responsible for the normal growth and development of the male sex organs and for maintenance of secondary sex characteristics. These effects include the growth and maturation of the prostate, seminal vesicles, penis, and scrotum; the development of male hair distribution, such as facial, pubic, chest, and axillary hair; laryngeal enlargement, vocal cord thickening, and alterations in body musculature and fat distribution.
Male hypogonadism, a clinical syndrome resulting from insufficient secretion of testosterone, has two main etiologies. Primary hypogonadism is caused by defects of the gonads, such as Klinefelter’s syndrome or Leydig cell aplasia, whereas secondary hypogonadism is the failure of the hypothalamus (or pituitary) to produce sufficient gonadotropins (FSH, LH).