Zonalon - Doxepin Hydrochloride cream
(Doxepin Hydrochloride)Zonalon - Doxepin Hydrochloride cream Prescribing Information
Zonalon® Cream is indicated for the short-term (up to 8 days) management of moderate pruritus in adult patients with atopic dermatitis or lichen simplex chronicus. (See DOSAGE AND ADMINISTRATION.)
A thin film of Zonalon® Cream should be applied four times each day with at least a 3 to 4 hour interval between applications. There are no data to establish the safety and effectiveness of Zonalon® Cream when used for greater than 8 days. Chronic use beyond eight days may result in higher systemic levels and should be avoided. Use of Zonalon® Cream for longer than 8 days may result in an increased likelihood of contact sensitization.
The risk for sedation may increase with greater body surface area application of Zonalon® Cream (See WARNINGS section). Clinical experience has shown that drowsiness is significantly more common in patients applying Zonalon® Cream to over 10% of body surface area; therefore, patients with greater than 10% of body surface area (see WARNINGS section) affected should be particularly cautioned concerning possible drowsiness and other systemic adverse effects of doxepin. If excessive drowsiness occurs, it may be necessary to do one or more of the following: reduce the body surface area treated, reduce the number of applications per day, reduce the amount of cream applied, or discontinue the drug.
Occlusive dressings may increase the absorption of most topical drugs; therefore, occlusive dressings should not be utilized with Zonalon® Cream.
Because doxepin HCl has an anticholinergic effect and because significant plasma levels of doxepin are detectable after topical Zonalon® Cream application, the use of Zonalon® Cream is contraindicated in patients with untreated narrow angle glaucoma or a tendency to urinary retention.
Zonalon® Cream is contraindicated in individuals who have shown previous sensitivity to any of its components.
In controlled clinical trials of patients treated with Zonalon® Cream, the most common systemic adverse event reported was drowsiness. Drowsiness occurred in 71 of 330 (22%) of patients treated with Zonalon® Cream compared to 7 of 334 (2%) of patients treated with vehicle cream. Drowsiness resulted in the premature discontinuation of the drug in approximately 5% of patients treated with Zonalon® Cream in controlled clinical trials.
Studies have not been performed examining drug interactions with Zonalon® Cream. However, since plasma levels of doxepin following topical application of Zonalon® Cream can reach levels obtained with oral doxepin HCl therapy, the following drug interactions are possible following topical Zonalon® Cream application:
Zonalon® (doxepin hydrochloride) Cream, 5% is a topical cream. Each gram contains: 50 mg of doxepin hydrochloride (equivalent to 44.3 mg of doxepin).
Doxepin hydrochloride, USP is one of a class of agents known as dibenzoxepin tricyclic antidepressant compounds. It is an isomeric mixture of N,N-dimethyldibenz[
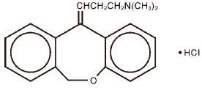
Zonalon® Cream also contains sorbitol, cetyl alcohol, isopropyl myristate, glyceryl stearate, PEG-100 stearate, petrolatum, benzyl alcohol, titanium dioxide and purified water.