Abrysvo
(respiratory syncytial virus pre-fusion F protein, A, recombinant, stabilized)Dosage & Administration
Abrysvo Prescribing Information
Immunization of Pregnant Individuals
ABRYSVO is a vaccine indicated for active immunization of pregnant individuals at 32 through 36 weeks gestational age for the prevention of lower respiratory tract disease (LRTD) and severe LRTD caused by respiratory syncytial virus (RSV) in infants from birth through 6 months of age.
Immunization of Individuals 60 Years of Age and Older
ABRYSVO is a vaccine indicated for active immunization for the prevention of LRTD caused by RSV in individuals 60 years of age and older.
Immunization of Individuals 18 Through 59 Years of Age
ABRYSVO is a vaccine indicated for active immunization for the prevention of LRTD caused by RSV in individuals 18 through 59 years of age who are at increased risk for LRTD caused by RSV.
Dose and Schedule
After reconstitution, a single dose of ABRYSVO is either 0.5 mL (Act-O-Vial presentation and vial and vial presentation) or approximately 0.5 mL (vial and prefilled syringe presentation) [see Dosage and Administration (2.2)].
Presentations and Reconstitution
ABRYSVO is supplied in 3 presentations as follows:
Act-O-Vial Presentation
The Act-O-Vial presentation is supplied in cartons. Each Act-O-Vial contains the Lyophilized Antigen Component (a sterile white powder) and Sterile Water Diluent Component.
Vial and Prefilled Syringe Presentation
The vial and prefilled syringe presentation is supplied in cartons containing a kit(s). Each kit includes a vial of Lyophilized Antigen Component (a sterile white powder), a prefilled syringe containing Sterile Water Diluent Component, and a vial adapter.
Vial and Vial Presentation
The vial and vial presentation is supplied in cartons that include vials of Lyophilized Antigen Component (a sterile white powder) and vials containing Sterile Water Diluent Component.
For all presentations, reconstitute the Lyophilized Antigen Component with the Sterile Water Diluent Component to form ABRYSVO, as described in the instructions below.
Reconstitution Instructions for the Act-O-Vial Presentation
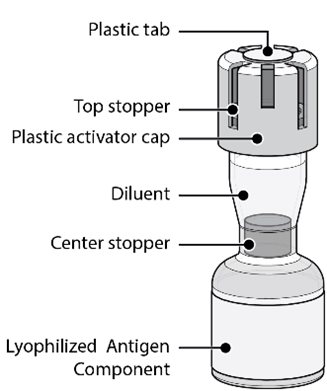 | 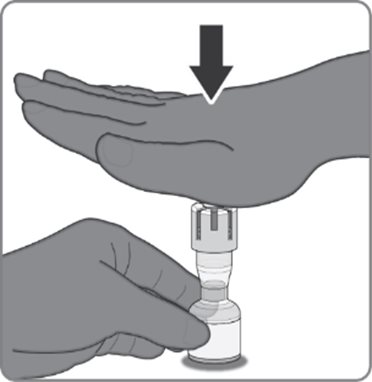 | 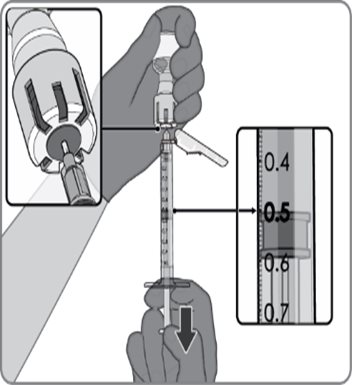 |
Act-O-Vial presentation | Step 1. Reconstitution of the Lyophilized Antigen Component with the Sterile Water Diluent Component to form ABRYSVO | Step 2. Withdrawal of ABRYSVO |
Reconstitution Instructions for Vial and Prefilled Syringe Presentation
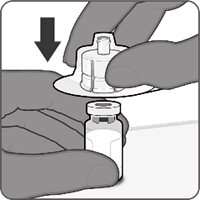 | 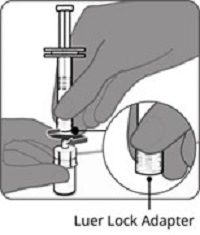 | 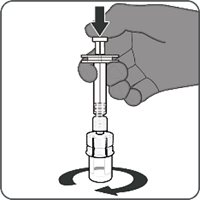 | 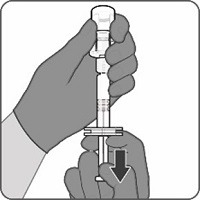 |
Step 1. Attachment of the vial adapter to the vial | Step 2. Connection of the syringe to the vial adapter | Step 3. Reconstitution of the Lyophilized Antigen Component with the Sterile Water Diluent Component to form ABRYSVO | Step 4. Withdrawal of ABRYSVO |
Reconstitution Instructions for the Vial and Vial Presentation
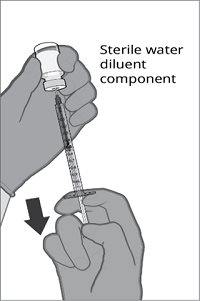 | 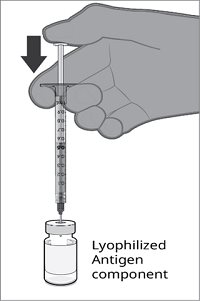 | 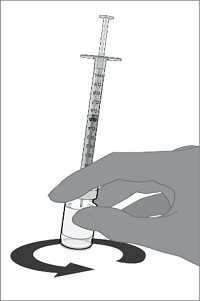 | 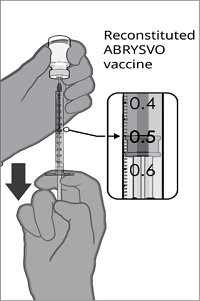 |
Step 1. Withdrawal of the Sterile Water Diluent | Step 2. Reconstitution of the Lyophilized Antigen Component with the Sterile Water Diluent Component to form ABRYSVO | Step 3. Withdrawal of ABRYSVO | |
Administration
For intramuscular injection
After reconstitution, ABRYSVO is a clear and colorless solution. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard if either condition is present.
Administer ABRYSVO immediately or store at room temperature [15°C to 30°C (59°F to 86°F)] and use within 4 hours. Discard reconstituted vaccine if not used within 4 hours.
For injection.
For the Act-O-Vial presentation, a single dose after reconstitution is 0.5 mL.
For the vial and prefilled syringe presentation, a single dose after reconstitution is approximately 0.5 mL.
For the vial and vial presentation, a single dose after reconstitution is 0.5 mL.
Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in individuals exposed to ABRYSVO during pregnancy. Individuals who received ABRYSVO during pregnancy are encouraged to contact, or have their healthcare provider contact, 1-800-616-3791 to enroll in or obtain information about the registry.
Risk Summary
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the US general population, the estimated background risk of major birth defects and miscarriages in clinically recognized pregnancies is 2% to 4%, and 15% to 20%, respectively, and the estimated background risk of fetal deaths after 20 weeks is 0.6%.
Study 1 enrolled 7,386 pregnant individuals who were randomized 1:1 and received ABRYSVO or placebo (0.5 mL dose, containing the same buffer ingredients in the same quantities as in a single dose of ABRYSVO [see Description (11)]) revealed no evidence for vaccine-associated increase in the risk of congenital anomalies or fetal deaths. Study 2 evaluated 115 pregnant individuals who received ABRYSVO and 117 who received placebo. A numerical imbalance in preterm births in ABRYSVO recipients was observed compared to placebo recipients in these two clinical studies. Available data are insufficient to establish or exclude a causal relationship between preterm birth and ABRYSVO [see Warnings and Precautions (5.2), Adverse Reactions (6.1), Clinical Considerations (8.1), Data (8.1) and Clinical Studies (14.1)].
A developmental toxicity study was performed in female rabbits administered a vaccine formulation containing two times the antigen content of a single human dose of ABRYSVO prior to and during gestation. The study showed no evidence of harm to the fetus or to postnatal survival, growth, or development (see Animal Data).
Clinical Considerations
Maternal Adverse Reactions
In Study 1, 3,698 pregnant individuals received ABRYSVO and 3,687 received placebo. Local and systemic adverse reactions occurred with greater frequency in the ABRYSVO group. Serious adverse reactions observed in pregnant individuals at a higher rate in the ABRYSVO group compared to the placebo group included pre-eclampsia (1.8% versus 1.4%) and gestational hypertension (1.2% versus 1.1%) [see Adverse Reactions (6.1)].
ABRYSVO has not been studied in pregnant individuals less than 24 weeks gestational age, and those at increased risk for preterm birth.
Fetal/Neonatal Adverse Reactions
The infant safety population included 3,659 and 3,646 infants born to individuals in the ABRYSVO or placebo group, respectively. There were 10 (0.3%) fetal deaths in the ABRYSVO group and 9 (0.2%) in the placebo group. Among the infants born to individuals in the ABRYSVO group and in the placebo group, 207 (5.7%) and 172 (4.7%), respectively, were born preterm [see Warnings and Precautions (5.2), Adverse Reactions (6.1) and Clinical Studies (14.1)]. Low birth weight was observed in 5.1% of participants in the ABRYSVO group versus 4.3% in the placebo group, and neonatal jaundice was observed in 7.3% in the ABRYSVO group versus 6.9% in the placebo group. [see Adverse Reactions (6.1)]. For mortality in the neonatal period among infants born to pregnant individuals in Study 1, there were 3 deaths in the ABRYSVO group and 5 in the placebo group, and for overall mortality including after the neonatal period there were 8 deaths in the ABRYSVO group and 12 in the placebo group. Congenital abnormalities were reported in 5.6% in the ABRYSVO group and 6.7% in the placebo group.
Available data are insufficient to establish or exclude a causal relationship between preterm birth and ABRYSVO. To avoid the potential risk of preterm birth with use of ABRYSVO before 32 weeks of gestation, administer ABRYSVO as indicated in pregnant individuals at 32 through 36 weeks gestational age.
Data
Human Data
In Study 1, 3,698 pregnant individuals received ABRYSVO and 3,687 received placebo at 24 through 36 weeks’ gestation. The infant safety population included 3,659 and 3,646 infants born to individuals in the ABRYSVO or placebo group, respectively. Among the infants born to individuals in the ABRYSVO group and in the placebo group, 207 (5.7%) and 172 (4.7%), respectively, had adverse events of preterm birth and 205 (5.6%) and 245 (6.7%), respectively, had reported congenital malformations or anomalies. There were 10 (0.3%) fetal deaths in the ABRYSVO group and 9 (0.2%) in the placebo group.
Animal Data
A pre- and post-natal developmental toxicity study with an embryo-fetal developmental toxicity phase was performed in female New Zealand White rabbits. Rabbits were administered 4 doses by intramuscular injection: at 3 weeks and at 1 week prior to mating, and on gestation days 10 and 24. On each occasion, rabbits received 0.5 mL of a vaccine formulation containing twice the antigen content of F glycoproteins of RSV A and RSV B (120 mcg RSV preF A and 120 mcg RSV preF B), stabilized in prefusion conformation as contained in a single human dose of ABRYSVO [see Description (11)]. No adverse effects on mating, female fertility, or on embryo/fetal or post-natal survival, growth, or development were observed. There were no vaccine-related fetal malformations or variations.
Lactation
Risk Summary
It is not known whether ABRYSVO is excreted in human milk. Data are not available to assess the effects of ABRYSVO on the breastfed infant or on milk production/excretion. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ABRYSVO and any potential adverse effects on the breastfed child from ABRYSVO or from the underlying maternal condition. For preventative vaccines, the underlying maternal condition is susceptibility to disease prevented by the vaccine.
Pediatric Use
The safety and effectiveness of ABRYSVO to prevent RSV LRTD and severe RSV LRTD in infants born to individuals vaccinated at younger than 10 years of age have not been established.
The safety and effectiveness of ABRYSVO to prevent RSV LRTD in non-pregnant individuals younger than 18 years of age via active immunization have not been established.
Geriatric Use
ABRYSVO is approved for use in individuals 60 years of age and older. In Study 3, of the 18,574 recipients who received ABRYSVO 63% (n=11,619) were aged 60-69 years of age, 32% (n=5,928) were 70-79 years of age and 5% (n=1,026) were ≥80 years of age [see Adverse Reactions (6.1) and Clinical Studies (14.2)].
Do not administer ABRYSVO to individuals with a history of a severe allergic reaction (e.g., anaphylaxis) to any component of ABRYSVO [see Description (11)].
GuillainBarré Syndrome
The results of a postmarketing observational study suggest an increased risk of Guillain‑Barré syndrome (GBS) during the 42 days following vaccination with ABRYSVO [see Adverse Reactions (6.2)].
Potential Risk of Preterm Birth
A numerical imbalance in preterm births in ABRYSVO recipients was observed compared to placebo recipients in two clinical studies [see Adverse Reactions (6.1)]. Available data are insufficient to establish or exclude a causal relationship between preterm birth and ABRYSVO. To avoid the potential risk of preterm birth with use of ABRYSVO before 32 weeks of gestation, administer ABRYSVO as indicated in pregnant individuals at 32 through 36 weeks gestational age. Pregnant individuals who were at increased risk of preterm birth were generally excluded from clinical studies of ABRYSVO.
Management of Acute Allergic Reactions
Appropriate medical treatment used to manage immediate allergic reactions must be immediately available in the event an anaphylactic reaction occurs following administration of ABRYSVO.
Syncope
Syncope (fainting) may occur in association with administration of injectable vaccines, including ABRYSVO. Procedures should be in place to avoid injury from fainting.
Altered Immunocompetence
Immunocompromised individuals, including those receiving immunosuppressive therapy, may have a diminished immune response to ABRYSVO.
Limitations of Vaccine Effectiveness
Vaccination with ABRYSVO may not protect all vaccine recipients.