Get your patient on Bimzelx (Bimekizumab)
Bimzelx prior authorization resources
Most recent state uniform prior authorization forms
Brand Resources
Bimzelx patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
- Prior to treatment: (2.1 )
- Evaluate patients for tuberculosis infection.
- Test liver enzymes, alkaline phosphatase, and bilirubin.
- Complete all age-appropriate vaccinations as recommended by current immunization guidelines.
- Plaque Psoriasis
- Administer 320 mg by subcutaneous injection at Weeks 0, 4, 8, 12, and 16, then every 8 weeks thereafter. For patients weighing 120 kg or more, consider a dose of 320 mg every 4 weeks after Week 16. (2.2 )
- Psoriatic Arthritis
- Non-Radiographic Axial Spondyloarthritis
- Administer 160 mg by subcutaneous injection every 4 weeks. (2.4 )
- Ankylosing Spondylitis
- Administer 160 mg by subcutaneous injection every 4 weeks. (2.5 )
- Hidradenitis Suppurativa
- Administer 320 mg by subcutaneous injection at Week 0, 2, 4, 6, 8, 10, 12, 14 and 16, then every 4 weeks thereafter. (2.6 )
- See full prescribing information for recommendations regarding missed doses, preparation and administration instructions. (2.7 , 2.8 , 2.9 )
Recommended Evaluations and Immunization Prior to Treatment Initiation
- Evaluate patients for tuberculosis (TB) infection prior to initiating treatment with BIMZELX [see Warnings and Precautions (5.3) ] .
- Test liver enzymes, alkaline phosphatase and bilirubin prior to initiating treatment with BIMZELX [see Warnings and Precautions (5.4) ] .
- Complete all age-appropriate vaccinations as recommended by current immunization guidelines [see Warning and Precautions (5.6) ] .
Recommended Dosage for Plaque Psoriasis
The recommended dosage is 320 mg by subcutaneous injection at Weeks 0, 4, 8, 12, and 16, then every 8 weeks thereafter . For patients weighing 120 kg or more, consider a dosage of 320 mg every 4 weeks after Week 16 [see Clinical Pharmacology (12.3) ] .
Recommended Dosage for Psoriatic Arthritis
The recommended dosage is 160 mg by subcutaneous injection every 4 weeks.
For psoriatic arthritis patients with coexistent moderate to severe plaque psoriasis, use the dosing regimen for adult patients with plaque psoriasis [see Dosage and Administration (2.2) ] .
Recommended Dosage for Non-Radiographic Axial Spondyloarthritis
The recommended dosage is 160 mg by subcutaneous injection every 4 weeks.
Recommended Dosage for Ankylosing Spondylitis
The recommended dosage is 160 mg by subcutaneous injection every 4 weeks.
Recommended Dosage for Hidradenitis Suppurativa
The recommended dosage is 320 mg by subcutaneous injection at Weeks 0, 2, 4, 6, 8, 10, 12, 14, and 16, then every 4 weeks thereafter.
Missed Doses
If a dose is missed, administer the dose as soon as possible. Thereafter, resume dosing at the regularly scheduled interval.
Preparation Instructions
- Before injecting, remove the carton with BIMZELX from the refrigerator and allow BIMZELX to reach room temperature (30 to 45 minutes) without removing the prefilled syringes or autoinjectors from the carton to protect from light.
- Inspect visually for particulate matter and discoloration prior to administration, whenever solution and container permit. BIMZELX injection is clear to slightly opalescent, and colorless to pale brownish- yellow. Do not use if the solution contains visible particles, is discolored or cloudy.
Administration Instructions
- BIMZELX is intended for use under the guidance and supervision of a healthcare professional. Patients may self-inject after training in subcutaneous injection technique. Provide proper training to patients and/or caregivers on the subcutaneous injection technique of BIMZELX according to the "Instructions for Use" [see Instructions for Use].
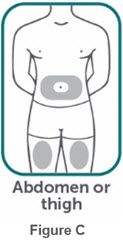
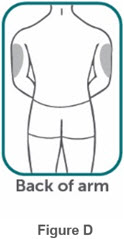
- If two separate 160 mg injections are used to achieve the recommended dose, administer each injection subcutaneously at a different anatomic location (such as thighs, abdomen or back of upper arm). Discard the syringes or autoinjectors after use. Do not reuse.
- Do not inject BIMZELX within 2 inches (5 cm) of the navel or into areas where the skin is tender, bruised, red, hard, thick, scaly, or affected by psoriasis. Administration of BIMZELX in the upper, outer arm may only be performed by a healthcare professional or caregiver. Rotate the injection site with each injection.
Coverage
See specific coverage requirements, including prior authorization and step therapies.

By using PrescriberAI, you agree to the AI Terms of Use.
Bimzelx prescribing information
INDICATIONS AND USAGE
BIMZELX is a humanized interleukin-17A and F antagonist indicated for the treatment of:
- Moderate to severe plaque psoriasis (PSO) in adults who are candidates for systemic therapy or phototherapy. (1.1 )
- Adults with active psoriatic arthritis (PsA) . (1.2 )
- Adults with active non-radiographic axial spondyloarthritis ( nr-axSpA ) with objective signs of inflammation. (1.3 )
- Adults with active ankylosing spondylitis ( AS ). (1.4 )
- Adults with moderate to severe hidradenitis suppurativa (HS) . (1.5 )
Plaque Psoriasis
BIMZELX is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Psoriatic Arthritis
BIMZELX is indicated for the treatment of adults with active psoriatic arthritis.
Non-Radiographic Axial Spondyloarthritis
BIMZELX is indicated for the treatment of adults with active non-radiographic axial spondyloarthritis with objective signs of inflammation.
Ankylosing Spondylitis
BIMZELX is indicated for the treatment of adults with active ankylosing spondylitis.
Hidradenitis Suppurativa
BIMZELX is indicated for the treatment of adults with moderate to severe hidradenitis suppurativa.
DOSAGE AND ADMINISTRATION
- Prior to treatment: (2.1 )
- Evaluate patients for tuberculosis infection.
- Test liver enzymes, alkaline phosphatase, and bilirubin.
- Complete all age-appropriate vaccinations as recommended by current immunization guidelines.
- Plaque Psoriasis
- Administer 320 mg by subcutaneous injection at Weeks 0, 4, 8, 12, and 16, then every 8 weeks thereafter. For patients weighing 120 kg or more, consider a dose of 320 mg every 4 weeks after Week 16. (2.2 )
- Psoriatic Arthritis
- Non-Radiographic Axial Spondyloarthritis
- Administer 160 mg by subcutaneous injection every 4 weeks. (2.4 )
- Ankylosing Spondylitis
- Administer 160 mg by subcutaneous injection every 4 weeks. (2.5 )
- Hidradenitis Suppurativa
- Administer 320 mg by subcutaneous injection at Week 0, 2, 4, 6, 8, 10, 12, 14 and 16, then every 4 weeks thereafter. (2.6 )
- See full prescribing information for recommendations regarding missed doses, preparation and administration instructions. (2.7 , 2.8 , 2.9 )
Recommended Evaluations and Immunization Prior to Treatment Initiation
- Evaluate patients for tuberculosis (TB) infection prior to initiating treatment with BIMZELX [see Warnings and Precautions (5.3) ] .
- Test liver enzymes, alkaline phosphatase and bilirubin prior to initiating treatment with BIMZELX [see Warnings and Precautions (5.4) ] .
- Complete all age-appropriate vaccinations as recommended by current immunization guidelines [see Warning and Precautions (5.6) ] .
Recommended Dosage for Plaque Psoriasis
The recommended dosage is 320 mg by subcutaneous injection at Weeks 0, 4, 8, 12, and 16, then every 8 weeks thereafter . For patients weighing 120 kg or more, consider a dosage of 320 mg every 4 weeks after Week 16 [see Clinical Pharmacology (12.3) ] .
Recommended Dosage for Psoriatic Arthritis
The recommended dosage is 160 mg by subcutaneous injection every 4 weeks.
For psoriatic arthritis patients with coexistent moderate to severe plaque psoriasis, use the dosing regimen for adult patients with plaque psoriasis [see Dosage and Administration (2.2) ] .
Recommended Dosage for Non-Radiographic Axial Spondyloarthritis
The recommended dosage is 160 mg by subcutaneous injection every 4 weeks.
Recommended Dosage for Ankylosing Spondylitis
The recommended dosage is 160 mg by subcutaneous injection every 4 weeks.
Recommended Dosage for Hidradenitis Suppurativa
The recommended dosage is 320 mg by subcutaneous injection at Weeks 0, 2, 4, 6, 8, 10, 12, 14, and 16, then every 4 weeks thereafter.
Missed Doses
If a dose is missed, administer the dose as soon as possible. Thereafter, resume dosing at the regularly scheduled interval.
Preparation Instructions
- Before injecting, remove the carton with BIMZELX from the refrigerator and allow BIMZELX to reach room temperature (30 to 45 minutes) without removing the prefilled syringes or autoinjectors from the carton to protect from light.
- Inspect visually for particulate matter and discoloration prior to administration, whenever solution and container permit. BIMZELX injection is clear to slightly opalescent, and colorless to pale brownish- yellow. Do not use if the solution contains visible particles, is discolored or cloudy.
Administration Instructions
- BIMZELX is intended for use under the guidance and supervision of a healthcare professional. Patients may self-inject after training in subcutaneous injection technique. Provide proper training to patients and/or caregivers on the subcutaneous injection technique of BIMZELX according to the "Instructions for Use" [see Instructions for Use].
- If two separate 160 mg injections are used to achieve the recommended dose, administer each injection subcutaneously at a different anatomic location (such as thighs, abdomen or back of upper arm). Discard the syringes or autoinjectors after use. Do not reuse.
- Do not inject BIMZELX within 2 inches (5 cm) of the navel or into areas where the skin is tender, bruised, red, hard, thick, scaly, or affected by psoriasis. Administration of BIMZELX in the upper, outer arm may only be performed by a healthcare professional or caregiver. Rotate the injection site with each injection.
DOSAGE FORMS AND STRENGTHS
- Injection (1 mL): 160 mg/mL clear to slightly opalescent, and colorless to pale brownish-yellow solution in a single-dose prefilled syringe or single-dose prefilled autoinjector.
- Injection (2 mL): 320 mg/2 mL (160 mg/mL) clear to slightly opalescent, and colorless to pale brownish-yellow solution in a single-dose prefilled syringe or single-dose prefilled autoinjector.
USE IN SPECIFIC POPULATIONS
Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to BIMZELX during pregnancy. For more information, healthcare providers or patients can contact the Organization of Teratology Information Specialists (OTIS) AutoImmune Diseases Study at 1-877-311-8972 or visit http://mothertobaby.org/pregnancy-studies/.
Risk Summary
Available data from case reports on BIMZELX use in pregnant women are insufficient to evaluate for a drug associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Transport of human IgG antibody across the placenta increases as pregnancy progresses and peaks during the third trimester; therefore, BIMZELX may be transmitted from the mother to the developing fetus (see Clinical Considerations ) . In an enhanced pre- and postnatal development study, no adverse developmental effects were observed in infants born to pregnant monkeys after subcutaneous administration of bimekizumab-bkzx during the period of organogenesis through parturition at doses up to 38 times the maximum recommended human dose (MRHD) (see Data ) .
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions: Because bimekizumab-bkzx may interfere with immune response to infections, risks and benefits should be considered prior to administering live vaccines to infants exposed to BIMZELX in utero. There are no data regarding infant serum levels of bimekizumab-bkzx at birth and the duration of persistence of bimekizumab-bkzx in infant serum after birth. Although a specific timeframe to delay live virus immunizations in infants exposed in utero is unknown, a minimum of 4 months after birth may be considered because of the half-life of the product.
Data
Animal Data: An enhanced pre- and postnatal developmental toxicity study was conducted in cynomolgus monkeys. Pregnant cynomolgus monkeys were administered subcutaneous doses of bimekizumab-bkzx of 20 or 50 mg/kg/week from gestation day 20 to parturition and the cynomolgus monkeys (mother and infants) were monitored for 6 months after delivery. No maternal toxicity was noted in this study. There were no treatment-related effects on growth and development, malformations, developmental immunotoxicology or neurobehavioral development. The no observed adverse effect level (NOAEL) for both maternal and developmental toxicity was identified as 50 mg/kg/week (38 times the MRHD, based on mg/kg comparison of 1.33 mg/kg/week administered as a 320 mg dose to a 60 kg individual once every 4 weeks).
Lactation
Risk Summary
There are no data on the presence of bimekizumab-bkzx in human or animal milk, the effects on the breastfed infant, or the effects on milk production. Endogenous IgG and monoclonal antibodies are transferred in human milk. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed infant to bimekizumab-bkzx are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for BIMZELX and any potential adverse effects on the breastfed infant from BIMZELX or from the underlying maternal condition.
Pediatric Use
The safety and effectiveness of BIMZELX in pediatric patients have not been established.
Geriatric Use
Of the 1,789 subjects with plaque psoriasis that were exposed to BIMZELX, a total of 153 subjects were 65 years of age or older, and 18 subjects were 75 years of age or older. Although no differences in safety or effectiveness were observed between subjects 65 years of age or older and younger adult subjects, clinical trials in PSO did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger adult subjects .
Of the 1,197 subjects with PsA that were exposed to BIMZELX, a total of 148 were 65 years of age and older. Although no differences in safety or effectiveness were observed between subjects 65 years of age or older and younger adult subjects, clinical trials in PsA did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger adult subjects .
Of the 244 subjects with nr-axSpA that were exposed to BIMZELX, a total of 6 were 65 years of age and older. Although no differences in safety or effectiveness were observed between subjects 65 years of age or older and younger adult subjects, the clinical trial in nr-axSpA did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger adult subjects .
Of the 330 subjects with AS that were exposed to BIMZELX, a total of 11 were 65 years of age and older. Although no differences in safety or effectiveness were observed between subjects 65 years of age or older and younger adult subjects, the clinical trial in AS did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger adult subjects .
Of the 995 subjects with hidradenitis suppurativa that were exposed to BIMZELX, a total of 18 were 65 years of age and older. Although no differences in safety or effectiveness were observed between subjects 65 years of age or older and younger adult subjects, clinical trials in HS did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger adult subjects .
CONTRAINDICATIONS
None.
WARNINGS AND PRECAUTIONS
- Suicidal Ideation and Behavior (SI/B) : May increase risk of SI/B. Advise patients, their caregivers, and families to monitor for the emergence or worsening of depression, suicidal ideation, or other mood changes. If such changes occur, instruct patients to promptly seek medical attention or call the National Suicide and Crisis Lifeline at 988. Carefully weigh risks and benefits of treatment with BIMZELX in patients with a history of severe depression and/or suicidal ideation or behavior. (5.1 )
- Infections : May increase risk of infection. Instruct patients to seek medical advice if signs or symptoms of clinically important infection occur. If such an infection develops, do not administer BIMZELX until the infection resolves. (5.2 )
- Tuberculosis (TB) : Avoid use in patients with active TB. Initiate treatment of latent TB prior to BIMZELX treatment. (5.3 )
- Liver Biochemical Abnormalities : Elevated serum transaminases were reported in clinical trials. Test liver enzymes, alkaline phosphatase, and bilirubin at baseline and according to routine patient management. Permanently discontinue use of BIMZELX in patients with causally - associated combined elevations of transaminases and bilirubin. (5.4 )
- Inflammatory Bowel Disease (IBD) : Cases of IBD were reported in clinical trials with IL-17 inhibitors, including BIMZELX. Avoid use of BIMZELX in patients with active IBD. Monitor patients for signs and symptoms of IBD and discontinue treatment if new onset or worsening of signs and symptoms occurs. (5.5 )
- Immunizations : Avoid the use of live vaccines in patients treated with BIMZELX. (5.6 )
Suicidal Ideation and Behavior
An increased incidence of new onset or worsening suicidal ideation and behavior was observed in subjects treated with BIMZELX. A causal association between treatment with BIMZELX and increased risk of suicidal ideation and behavior has not been definitively established.
Suicidal ideation and behavior were prospectively monitored using the Columbia Suicide Severity Rating Scale (C-SSRS) in clinical trials. The C-SSRS is an interview-based instrument used to monitor for the presence and severity of suicidal ideation (ranging from "none" to "active suicidal ideation with specific plan and intent") and behaviors (rating the injury and potential lethality of self-injury, if present).
Plaque Psoriasis
During the two 16-week, placebo-controlled periods of Trials Ps-1 and Ps-2, higher rates of suicidal ideation as assessed by C-SSRS were reported in BIMZELX-treated subjects than in subjects receiving placebo. Pooled analysis of C-SSRS data indicated that 12/670 (1.8%) BIMZELX-treated subjects and 1/169 (0.6%) subjects receiving placebo reported passive suicidal ideation with an estimated relative risk of 3.0 (95% confidence interval: 0.39, 22.74). Subjects without a prior history of SI/B treated with BIMZELX also reported a higher rate of new onset suicidal ideation on the C-SSRS than subjects receiving placebo (1.3% vs 0.6%). During the open-label extension trial, one completed suicide was reported in a BIMZELX-treated subject [see Adverse Reactions (6.1) ].
Psoriatic Arthritis
Pooled analysis of C-SSRS data from the two 16-week, placebo-controlled periods of Trials PsA-1 and PsA-2 indicated that 2/698 (0.3%) BIMZELX-treated subjects and 3/413 (0.7%) subjects receiving placebo reported passive suicidal ideation with an estimated relative risk of 0.35 (95% confidence interval: 0.05, 2.29) [see Adverse Reactions (6.1) ] .
Non-Radiographic Axial Spondyloarthritis
Analysis of C-SSRS data from a 16-week, placebo-controlled period of Trial nr-axSpA-1 indicated that no subjects, being treated either with BIMZELX or placebo, reported suicidal ideation [see Adverse Reactions (6.1) ] .
Ankylosing Spondylitis
Analysis of C-SSRS data from a 16-week, placebo-controlled period of Trial AS-1 indicated that no subjects, being treated either with BIMZELX or placebo, reported suicidal ideation [see Adverse Reactions (6.1) ] .
Hidradenitis Suppurativa
During the two 16-week, placebo-controlled periods of Trials HS-1 and HS-2, higher rates of suicidal ideation as assessed by C-SSRS were reported in BIMZELX-treated subjects than in subjects receiving placebo. Based on a pooled analysis of the first 16 weeks of the placebo controlled clinical trials, 16/861 subjects in the BIMZELX group (1.9 %) reported suicidal ideation on the C-SSRS compared to 1/146 subjects in the placebo group (0.7%) with an estimated relative risk of 2.70 (95% confidence interval: 0.36, 20.12). Subjects without a prior history of SI/B treated with BIMZELX also reported a higher rate of new-onset suicidal ideation on the C-SSRS than subjects treated with placebo (0.9% vs. 0%). [see Adverse Reactions 6.1 ].
Consider the potential risks and benefits before prescribing BIMZELX to patients with a history of severe depression or suicidal ideation or behavior. Advise patients, their caregivers, and families to monitor for the emergence or worsening of depression, suicidal ideation, or other mood changes. If such changes occur, instruct patients to promptly seek medical attention or call the National Suicide and Crisis Lifeline at 988 [see Patient Counseling Information (17) ] . Refer BIMZELX-treated patients with new or worsening symptoms of depression or suicidal ideation and/or behavior to a mental health professional, as appropriate. Re-evaluate the risks and benefits of continuing treatment with BIMZELX if such events occur.
Infections
BIMZELX may increase the risk of infections, including serious infections.
Do not initiate treatment with BIMZELX in patients with any clinically important active infection until the infection resolves or is adequately treated.
In patients with a chronic infection or a history of recurrent infection, consider the risks and benefits prior to prescribing BIMZELX. Instruct patients to seek medical advice if signs or symptoms of clinically important infection occur. If a patient develops such an infection or is not responding to standard therapy, monitor the patient closely and discontinue BIMZELX until the infection resolves.
Tuberculosis
Evaluate patients for tuberculosis (TB) infection prior to initiating treatment with BIMZELX. Avoid the use of BIMZELX in patients with active TB infection. Initiate treatment of latent TB prior to administering BIMZELX. Consider anti-TB therapy prior to initiation of BIMZELX in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed. Closely monitor patients treated with BIMZELX for signs and symptoms of active TB during and after treatment.
Liver Biochemical Abnormalities
Treatment with BIMZELX was associated with increased incidence of liver enzyme elevations compared to treatment with placebo in randomized clinical trials. Liver serum transaminase elevations > 3 times the upper limit of normal were reported in subjects treated with BIMZELX [see Adverse Reactions (6.1) ] . Elevated liver serum transaminases resolved after discontinuation of BIMZELX.
Test liver enzymes, alkaline phosphatase, and bilirubin at baseline, periodically during treatment with BIMZELX and according to routine patient management. If treatment-related increases in liver enzymes occur and drug-induced liver injury is suspected, interrupt BIMZELX until a diagnosis of liver injury is excluded. Permanently discontinue BIMZELX in patients with causally associated combined elevations of transaminases and bilirubin. Patients with acute liver disease or cirrhosis may be at increased risk for severe hepatic injury; avoid use of BIMZELX in these patients.
Inflammatory Bowel Disease
Cases of inflammatory bowel disease (IBD) have been reported in patients treated with IL-17 inhibitors, including BIMZELX [see Adverse Reactions (6.1) ]. Avoid use of BIMZELX in patients with active IBD. During BIMZELX treatment, monitor patients for signs and symptoms of IBD and discontinue treatment if new onset or worsening of signs and symptoms occurs.
Immunizations
Prior to initiating therapy with BIMZELX, complete all age-appropriate vaccinations according to current immunization guidelines. Avoid the use of live vaccines in patients treated with BIMZELX. Limited data are available regarding coadministration of BIMZELX with non-live vaccines [see Clinical Pharmacology (12.2) ] .
ADVERSE REACTIONS
The following adverse reactions have been observed with BIMZELX and are discussed in greater detail in other sections of the labeling:
- Suicidal Ideation and Behavior [see Warnings and Precautions (5.1) ]
- Infections [see Warnings and Precautions (5.2) ]
- Liver Biochemical Abnormalities [see Warnings and Precautions (5.4) ]
- Inflammatory Bowel Disease [see Warnings and Precautions (5.5) ]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Plaque Psoriasis Clinical Trials
In clinical trials, a total of 1,789 subjects with plaque psoriasis were treated with BIMZELX. Of these, 1,073 subjects were exposed to BIMZELX for at least one year.
The safety of BIMZELX was evaluated in two placebo-controlled trials (Ps-1 and Ps-2), an active-controlled trial (Ps-3), and an open-label extension trial. Data from Trials Ps-1 and Ps-2 in 839 subjects (mean age 45 years, 72% male, 84% White) were pooled to evaluate the safety of BIMZELX in comparison to placebo up to 16 weeks after treatment initiation. A total of 670 subjects were treated during this initial period with BIMZELX 320 mg at Weeks 0, 4, 8, 12, and 16. Table 1 summarizes the adverse reactions that occurred at a rate of 1% or greater and at a higher rate in the BIMZELX group than the placebo group.
| BIMZELX N=670 n (%) | Placebo N=169 n (%) | |
|---|---|---|
| URI Upper Respiratory Infections include nasopharyngitis, upper respiratory tract infection, pharyngitis, rhinitis, viral upper respiratory tract infection, tonsillitis, sinusitis, pharyngitis streptococcal, pharyngitis bacterial, peritonsillar abscess, viral rhinitis, and influenza | 102 (15) | 24 (14) |
| Oral Candidiasis Oral Candidiasis includes oral candidiasis, oropharyngeal candidiasis, oral fungal infection, fungal pharyngitis, and oropharyngitis fungal | 61 (9) | 0 (0) |
| Headache | 22 (3) | 0 (0) |
| Injection Site Reactions Injection Site Reactions include injection site reaction, injection site erythema, injection site pain, injection site edema, injection site bruising, and injection site swelling | 19 (3) | 2 (1) |
| Tinea Infections Tinea Infections include tinea pedis, fungal skin infection, tinea versicolor, tinea cruris, tinea infection, body tinea, and onychomycosis | 18 (3) | 1 (1) |
| Gastroenteritis Gastroenteritis includes Enterovirus infection, gastroenteritis, gastroenteritis bacterial, and gastroenteritis viral | 12 (2) | 0 (0) |
| Herpes Simplex Infections Herpes Simplex Infections include herpes simplex and oral herpes | 9 (1) | 0 (0) |
| Acne | 8 (1) | 0 (0) |
| Folliculitis | 8 (1) | 0 (0) |
| Other Candida Infections Other Candida Infections include vulvovaginal candidiasis, vulvovaginal mycotic infection, skin candida, and genital candidiasis. | 7 (1) | 1 (1) |
| Fatigue | 7 (1) | 0 (0) |
Adverse reactions that occurred in < 1% but > 0.1% of subjects in the BIMZELX group and at a higher rate than in the placebo group through Week 16 were neutropenia, eczema, otitis externa, otitis media, and pyrexia.
The safety of BIMZELX was evaluated in another active-controlled trial (Ps-4) in 743 adult subjects who received BIMZELX 320 mg every 4 weeks or every 8 weeks through Week 48.
Specific Adverse Reactions
Suicidal Ideation and Behavior : The study populations of Trial Ps-1, Trial Ps-2, Trial Ps-3 and Trial Ps-4 excluded subjects with active suicidal ideation, suicidal ideation within the month prior to screening, a history of suicide attempt within the past 5 years prior to screening, or moderately severe to severe major depression (i.e., score of ≥15 on the screening Patient Health Questionnaire-9 (PHQ-9)).
Analysis of pooled C-SSRS data from the first 16 weeks of placebo-controlled clinical trials indicated that 12/670 (1.8%) BIMZELX-treated subjects and 1/169 (0.6%) subjects receiving placebo reported passive suicidal ideation with an estimated relative risk of 3.0 (95% confidence interval: 0.39, 22.74). Subjects without a prior history of SI/B treated with BIMZELX also reported a higher rate of new onset suicidal ideation on the C-SSRS than subjects receiving placebo (1.3% vs 0.6%).
During the course of the clinical trials for plaque psoriasis, there was 1 completed suicide in the open label extension trial after 718 days of treatment (1/2,480; 0.01/100 patient-years). The completed suicide was reported in a subject without a past reported psychiatric history. There were also 3 suicide attempts (3/2,480; 0.04/100 patient-years); 2 of these subjects had a history of prior suicide attempts.
Infections : During the placebo-controlled period of Trials Ps-1 and Ps-2, infections were reported in 36% of subjects (141.7 per 100 patient-years) treated with BIMZELX compared with 23% of subjects (84.6 per 100 patient-years) receiving placebo. Serious infections occurred in 0.3% of subjects (1.0 per 100 patient-years) treated with BIMZELX and 0% subjects receiving placebo .
The most common infections were upper respiratory tract infections and Candida infections, including oral candidiasis (oral candidiasis, oropharyngeal candidiasis, oral fungal infection, fungal pharyngitis, and oropharyngitis fungal) occurring in 9% (30.6 per 100 patient-years) of subjects treated with BIMZELX and other Candida infections (vulvovaginal candidiasis, vulvovaginal mycotic infection, skin candida, and genital candidiasis) in 1% (3.4 per 100 patient-years) of subjects treated with BIMZELX compared to 0% and 1%, respectively, of subjects receiving placebo.
During the combined initial, maintenance, and open-label extension treatment periods of trials Ps-1, Ps-2, Ps-3, and the open-label extension trial, infections were reported in 63% of subjects treated with BIMZELX (120.4 per 100 patient-years). Serious infections were reported in 1.5% of subjects treated with BIMZELX (1.6 per 100 patient-years).
Inflammatory Bowel Disease : In clinical trials in subjects with plaque psoriasis, subjects with active inflammatory bowel disease were excluded. In these trials, which included 2,480 subjects exposed to BIMZELX accounting for 5,830 patient-years, adjudicated cases of new onset of inflammatory bowel disease (including ulcerative colitis (UC), Crohn's disease (CD) and IBD-undetermined) occurred in seven subjects (0.12 per 100 patient-years); the majority of these cases were serious and resulted in discontinuation of therapy.
Liver Biochemical Abnormalities : During the placebo-controlled period of Trials Ps-1 and Ps-2, liver serum transaminase elevations (> 3 times the upper limit of normal [ULN]) occurred in 1.0% of subjects treated with BIMZELX versus 0.6% of subjects receiving placebo. The time to onset of these adverse reactions varied between 28 and 198 days after starting BIMZELX treatment. Elevated liver serum transaminases resolved during continued treatment or after discontinuation of BIMZELX.
Safety through Week 56
During the maintenance period (Week 16 through Week 52 of Trial Ps-1 and Week 56 of Trial Ps-2), adverse reactions were consistent with those observed during the initial 16 weeks of treatment with BIMZELX. During the maintenance treatment periods of Trial Ps-2 and Trial Ps-3, the rates of adverse reactions were similar between subjects treated with BIMZELX 320 mg every four week and every eight weeks, after the initial 16 weeks of treatment.
Safety through Week 128
During the open-label extension trial, including data from Week 56 through Week 128, new adverse reactions of suicide attempt and a completed suicide occurred [described above in Suicidal Ideation and Behavior ].
Additional Safety Data
In an active-controlled clinical trial (Trial Ps-4), 691 subjects with plaque psoriasis were treated with BIMZELX for up to 144 weeks. Adverse reactions were consistent with those observed during the initial 16 weeks of treatment and with the overall safety profile of BIMZELX.
Psoriatic Arthritis Clinical Trials
The safety of BIMZELX was evaluated in two placebo-controlled trials (PsA-1 and PsA-2). Data from Trials PsA-1 and PsA-2 in 1,111 subjects (mean age 49 years, 47% male, 96% White) were pooled to evaluate the safety of BIMZELX in comparison to placebo up to 16 weeks after treatment initiation. A total of 698 subjects were treated during this initial period with BIMZELX 160 mg at Weeks 0, 4, 8, 12, and 16. Table 2 summarizes the adverse reactions that occurred at a rate of 2% or greater and at a higher rate in the BIMZELX group than the placebo group.
| BIMZELX N=698 n (%) | Placebo N=413 n (%) | |
|---|---|---|
| URI Upper Respiratory Tract Infections (URI) includes: nasopharyngitis, upper respiratory tract infection, pharyngitis, sinusitis, and rhinitis. | 99 (14) | 41 (10) |
| Headache | 25 (4) | 7 (2) |
| Diarrhea | 19 (3) | 8 (2) |
| Urinary Tract Infection | 14 (2) | 7 (2) |
| Oral Candidiasis | 16 (2) | 0 |
Adverse reactions that occurred in < 2% but > 1% of subjects in the BIMZELX group and at a higher rate than in the placebo group through Week 16 included neutropenia (placebo: n=0; BIMZELX: n=8 (1.1%)), stomatitis (placebo: n=0; BIMZELX: n=8 (1.1%)), bronchitis (placebo: n=1 (0.2%); BIMZELX: n=11 (1.6%)), and oropharyngeal pain (placebo: n=0; BIMZELX: n=9 (1.3%)).
Specific Adverse Reactions
Suicidal Ideation and Behavior: The neuropsychiatric inclusion/exclusion criteria in PsA trials were the same as in PSO.
Based on a pooled analysis of the first 16 weeks of the placebo controlled clinical trials, 2 of the 698 subjects in the BIMZELX group (0.3%) reported passive suicidal ideation on the C-SSRS compared to 3 of 413 subjects in the placebo group (0.7%).
During the entire clinical trial program for PsA (2,664 patient-years), there were 2 cases of suicidal ideation (2/1413; 0.08/100 patient-years) and 1 suicide attempt (1/1413; 0.04/100 patient-years); all reported in BIMZELX-treated subjects with pre-existing psychiatric conditions.
Infections: During the placebo-controlled period of Trials PsA-1 and PsA-2, infections were reported in 27% of subjects (100.7 per 100 patient-years) treated with BIMZELX compared with 18% of subjects (62.8 per 100 patient-years) treated with placebo. Serious infections occurred in 0.4% of subjects (1.4 per 100 patient-years) treated with BIMZELX and 0% treated with placebo .
The most common infections were upper respiratory tract infections, nasopharyngitis, urinary tract infection and Candida infections, including oral candidiasis (oral candidiasis, oral fungal infection, and tongue fungal infection), occurring in 3.2% (10.2 per 100 patient-years) of subjects treated with BIMZELX and other Candida infections (skin candida, vulvovaginal candidiasis, and vulvovaginal mycotic infection) in 0.6% (1.8 per 100 patient-years) of subjects treated with BIMZELX compared to 0% and 1%, respectively, of subjects treated with placebo.
During the combined placebo-controlled, maintenance and open-label extension treatment periods of Trials PsA-1 and PsA-2, infections were reported in 58% of subjects treated with BIMZELX (58.5 per 100 patients-years). Serious infections were reported in 2% of subjects treated with BIMZELX (1.3 per 100 patient-years).
Inflammatory Bowel Disease: In clinical trials in subjects with psoriatic arthritis, subjects with active inflammatory bowel disease were excluded. In these trials, which included 1,413 subjects exposed to BIMZELX accounting for 2,664 patient-years, adjudicated cases of new onset of inflammatory bowel disease (including ulcerative colitis (UC) and IBD) occurred in 2 subjects (0.08 per 100 patient-years); one of these cases was serious and none resulted in discontinuation of therapy.
Liver Biochemical Abnormalities: During the placebo-controlled period of Trials PsA-1 and PsA-2, liver serum transaminase elevations (> 3 times the upper limit of normal [ULN]) occurred in 1.3% of subjects treated with BIMZELX versus 0% of subjects receiving placebo. Elevated liver serum transaminases resolved during continued treatment or after discontinuation of BIMZELX.
Safety through Week 52
During the maintenance period (Week 16 through Week 52 of Trial PsA-1), adverse reactions were consistent with those observed during the initial 16 weeks of treatment and with the overall safety profile of BIMZELX.
Non-Radiographic Axial Spondyloarthritis Clinical Trials
BIMZELX was evaluated in a placebo-controlled trial (Trial nr-axSpA-1) in subjects with non-radiographic axial spondyloarthritis (128 subjects on BIMZELX and 126 subjects on placebo). The safety profile observed in subjects with non-radiographic axial spondyloarthritis treated with BIMZELX was overall similar to the safety profile seen in subjects with psoriatic arthritis, except for cough, musculoskeletal pain, myalgia, tonsilitis, transaminase increase (placebo: n=0; BIMZELX: n=3 (2.3%) for each), and fatigue (placebo: n=1 (0.8%); BIMZELX: n=3 (2.3%)).
Specific Adverse Reactions
Suicidal Ideation and Behavior: The neuropsychiatric inclusion/exclusion criteria were the same in non-radiographic axial spondyloarthritis trials as in PSO.
During the first 16 weeks of the placebo controlled clinical trial (Trial nr-axSpA-1), no subjects in the BIMZELX or placebo group reported suicidal ideation on the C-SSRS. During the entire clinical trial program for nr-axSpA (398 patient-years), there were no cases of suicidal ideations. One suicide attempt (1/244; 0.25/100 patient-years) was reported in a BIMZELX-treated patient with pre-existing psychiatric conditions and recent life stressors.
Infections: During the placebo-controlled period of Trial nr-axSpA-1, infections were reported in 36% of subjects (144.8 per 100 patient-years) treated with BIMZELX compared with 25% of subjects (94.4 per 100 patient-years) receiving placebo. There were no reports of serious infections reported during the placebo-controlled period of the trial.
During the combined 52 week treatment period of Trial nr-axSpA-1, and subsequent open-label treatment, infections were reported in 68% of subjects treated with BIMZELX (78.0 per 100 patient-years). Serious infections were reported in 1.6% of subjects treated with BIMZELX (1.0 per 100 patient-years).
Inflammatory Bowel Disease: In the clinical trial in subjects with non-radiographic axial spondyloarthritis, subjects with active inflammatory bowel disease were excluded. In placebo-controlled, maintenance, and open label treatment periods of this trial, which included 244 subjects exposed to BIMZELX accounting for 397 patient-years, adjudicated cases of new onset of inflammatory bowel disease occurred in 1 subject (Ulcerative Colitis; 0.26 per 100 patient-years); this case of ulcerative colitis was nonserious and did not result in discontinuation of therapy.
Liver Biochemical Abnormalities: During the placebo-controlled period of Trials nr-axSpA-1, liver serum transaminase elevations (> 3 times the upper limit of normal [ULN]) occurred in 1.6% of subjects treated with BIMZELX versus 0.8% of subjects receiving placebo. Elevated liver serum transaminases resolved during continued treatment or after discontinuation of BIMZELX.
Safety through Week 52
During the maintenance period (Week 16 through Week 52 of Trial nr-axSpA-1), adverse reactions were consistent with those observed during the initial 16 weeks of treatment and with the overall safety profile of BIMZELX.
Ankylosing Spondylitis Clinical Trials
BIMZELX was evaluated in a placebo-controlled trial (Trial AS-1) in subjects with ankylosing spondylitis (221 subjects on BIMZELX and 111 subjects on placebo). The safety profile observed in subjects with ankylosing spondylitis treated with BIMZELX was overall similar to the safety profile seen in subjects with psoriatic arthritis, except for injection site pain, rash (placebo: n=1 (0.9%); BIMZELX: n=6 (2.7%), for each) and vulvovaginal mycotic infection (placebo: n=0; BIMZELX: n=5 (2.3%)).
Specific Adverse Reactions
Suicidal Ideation and Behavior: The neuropsychiatric inclusion/exclusion criteria were the same in AS trials as in PSO.
During the first 16 weeks of the placebo controlled clinical Trial AS-1, no subjects in the BIMZELX or placebo group reported suicidal ideation on the C-SSRS. During the entire clinical trial program for AS (1,844 patient-years), there was 1 case of suicidal ideation (1/684; 0.05/100 patient-years) reported in a subject with pre-existing psychiatric conditions.
Infections: During the placebo-controlled period of Trial AS-1, infections were reported in 28% of subjects (110.3 per 100 patient-years) treated with BIMZELX compared with 23% of subjects (83.7 per 100 patient-years) receiving placebo. Serious infections occurred in 1 (0.5%) subject (1.5 per 100 patient-years) treated with BIMZELX and 1 (0.9%) subject (2.9 per 100 patient-years) receiving placebo .
During the combined 52 week treatment period of Trial AS-1, and subsequent open-label treatment, infections were reported in 62% of subjects treated with BIMZELX (58.8 per 100 patient-years). Serious infections were reported in 2.7% of subjects treated with BIMZELX (1.5 per 100 patient-years).
Inflammatory Bowel Disease: In clinical trials in subjects with AS, subjects with active inflammatory bowel disease were excluded. In these phase 2/3 trials, which included 593 subjects exposed to BIMZELX accounting for 1,599 patient-years, adjudicated cases of new onset of inflammatory bowel disease (including ulcerative colitis (UC), Crohn's disease (CD) and IBD-undetermined) occurred in 6 subjects (0.38 per 100 patient-years); 4 cases were serious, and 3 cases resulted in discontinuation of therapy.
Liver Biochemical Abnormalities: During the placebo-controlled period of Trial AS-1, liver serum transaminase elevations (> 3 times the upper limit of normal [ULN]) occurred in 1.4% of subjects treated with BIMZELX versus 1.8% of subjects receiving placebo. Elevated liver serum transaminases resolved during continued treatment or after discontinuation of BIMZELX.
Safety through Week 52
During the maintenance period (Week 16 through Week 52 of Trial AS-1), adverse reactions were consistent with those observed during the initial 16 weeks of treatment and with the overall safety profile of BIMZELX.
Hidradenitis Suppurativa Clinical Trials
BIMZELX was evaluated in two placebo-controlled trials (Trial HS-1 and Trial HS-2) in 1,007 adult subjects with moderate to severe hidradenitis suppurativa (861 BIMZELX-treated subjects and 146 subjects receiving placebo) [see Clinical Studies (14.5) ] . The safety profile observed in subjects with hidradenitis suppurativa treated with BIMZELX was overall similar to the safety profile seen in subjects with PSO treated with BIMZELX.
Upon completion of both trials, a total of 657 subjects enrolled in a long-term extension treatment period for up to an additional 188 weeks.
Specific Adverse Reactions
Suicidal Ideation and Behavior : The neuropsychiatric inclusion/exclusion criteria were the same in HS trials as in PSO.
Analysis of pooled C-SSRS data from the first 16 weeks of the placebo-controlled clinical trials indicated that 16/861 (1.9%) BIMZELX-treated subjects and 1/146 (0.7%) subjects receiving placebo reported suicidal ideation with an estimated relative risk of 2.70 (95% confidence interval: 0.36, 20.12). Subjects without a prior history of SI/B treated with BIMZELX also reported a higher rate of new-onset suicidal ideation on the C-SSRS than subjects treated with placebo (0.9% vs. 0%).
There were 2 reported cases of suicidal ideation that were adjudicated as suicide attempts (2/1,041; 0.15/100 patient-years). Both subjects had a history of neuropsychiatric disorders.
Infections: During the placebo-controlled period of Trials HS-1 and HS-2, infections were reported in 33% of subjects (132.2 per 100 patient-years) treated with BIMZELX compared with 21% of subjects (76.4 per 100 patient-years) receiving placebo. Serious infections occurred in 0.1% of subjects (0.4 per 100 patient-years) treated with BIMZELX and 0% of subjects receiving placebo .
The most commonly reported infections were comparable to those reported in subjects with PSO. Oral candidiasis occurred in 7.8% of subjects (26.7 per 100 patient years) treated with BIMZELX 320 mg Q2W, 3.9% of subjects (12.9 per 100 patient-years) treated with BIMZELX 320 mg Q4W, and 0 subjects receiving placebo. Other candida infections occurred in 3.6% of subjects (12.2 per 100 patient-years) treated with BIMZELX 320 mg Q2W, 6.7% of subjects (22.6 per 100 patient-years) treated with BIMZELX 320 mg Q4W, and 0 subjects receiving placebo. Tinea infections occurred in 3.1% of subjects (10.4 per 100 patient-years) treated with BIMZELX 320 mg Q2W, 2.5% of subjects (8.1 per 100 patient-years) treated with BIMZELX 320 mg Q4W, and 0.7% (2.3 per 100 patient-years) receiving placebo.
During the combined placebo-controlled, maintenance treatment periods and open-label extension treatment periods of Trials HS-1 and HS-2, infections were reported in 68% of subjects treated with BIMZELX (104.7 per 100 patient-years). Serious infections were reported in 2.1% of subjects treated with BIMZELX (1.7 per 100 patient-years).
Inflammatory Bowel Disease: In clinical trials in subjects with hidradenitis suppurativa, subjects with active inflammatory bowel disease were excluded. During the combined placebo-controlled, maintenance, and open-label extension treatment periods of Trials HS-1 and HS-2, which included 995 subjects exposed to BIMZELX accounting for 1,272 patient-years, adjudicated cases of new onset of inflammatory bowel disease (including ulcerative colitis (UC), Crohn's disease (CD) and undetermined) occurred in 5 subjects (0.39 per 100 patient-years); 3 of these cases were serious, all of which resulted in discontinuation of therapy. Additionally, flares of pre-existing IBD occurred in 2 subjects, which resulted in discontinuation of BIMZELX in both.
Liver Biochemical Abnormalities: During the placebo-controlled period of Trials HS-1 and HS-2, liver serum transaminase elevations (> 3 times the upper limit of normal [ULN]) occurred in 1.2% of subjects treated with BIMZELX versus 0% of subjects receiving placebo. Elevated liver serum transaminases resolved during continued treatment or after discontinuation of BIMZELX.
Safety through Week 48
During the maintenance period (Week 16 through Week 48 of Trials HS-1 and HS-2), adverse reactions were consistent with those observed during the initial 16 weeks of treatment and with the overall safety profile of BIMZELX.
Postmarketing Experience
The following adverse reactions have been reported during post-approval use of BIMZELX. Because they are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Infections: conjunctivitis, esophageal candidiasis
DRUG INTERACTIONS
CYP450 Substrates
The formation of CYP450 enzymes can be altered by increased levels of certain cytokines (e.g., IL-1, IL-6, IL-10, TNFα, IFN) during chronic inflammation. Treatment with BIMZELX may modulate serum levels of some cytokines.
Therefore, upon initiation or discontinuation of BIMZELX in patients who are receiving concomitant drugs which are CYP450 substrates, particularly those with a narrow therapeutic index, consider monitoring for effect (e.g., for warfarin) or drug concentration (e.g., for cyclosporine) and consider dosage modification of the CYP450 substrate.
Population pharmacokinetic (PK) data analyses indicated that the clearance of BIMZELX was not impacted by concomitant administration of cDMARDs including methotrexate, or by prior exposure to biologics.
DESCRIPTION
Bimekizumab-bkzx, an interleukin-17 A and F antagonist, is a recombinant humanized immunoglobulin G1 (IgG1) monoclonal antibody. Bimekizumab-bkzx is produced by recombinant DNA technology in Chinese Hamster Ovary cells, and has an approximate molecular weight of 150 kDa.
BIMZELX (bimekizumab-bkzx) injection is a sterile, preservative-free, clear to slightly opalescent, and colorless to pale brownish-yellow solution for subcutaneous use.
Each BIMZELX 1 mL (160 mg/mL) prefilled syringe or prefilled autoinjector delivers 1 mL containing 160 mg bimekizumab-bkzx, glacial acetic acid (1.23 mg), glycine (16.5 mg), polysorbate 80 (0.4 mg), sodium acetate (2.83 mg), and Water for Injection, USP at pH 5.1.
Each BIMZELX 2 mL (160 mg/mL) prefilled syringe or prefilled autoinjector delivers 2 mL containing 320 mg bimekizumab-bkzx, glacial acetic acid (2.46 mg), glycine (33.0 mg), polysorbate 80 (0.8 mg), sodium acetate (5.65 mg), and Water for Injection, USP at pH 5.1.
CLINICAL PHARMACOLOGY
Mechanism of Action
Bimekizumab-bkzx is a humanized immunoglobulin IgG1/ κ monoclonal antibody with two identical antigen binding regions that selectively bind to human interleukin 17A (IL-17A), interleukin 17F (IL-17F), and interleukin 17-AF cytokines, and inhibits their interaction with the IL-17 receptor complex. IL-17A and IL-17F are naturally occurring cytokines that are involved in normal inflammatory and immune responses. Bimekizumab-bkzx inhibits the release of proinflammatory cytokines and chemokines.
Pharmacodynamics
Elevated levels of IL-17A and IL-17F are found in lesional psoriatic skin, and lesional skin in HS. Bimekizumab-bkzx exposure-response relationships to serum biomarkers, including IL-17A and IL-17F, and the time course of such pharmacodynamic responses are unknown.
Immune Response to Inactivated or Non-Live Vaccines
Healthy individuals who received a single 320 mg dose of BIMZELX two weeks prior to vaccination with an inactivated seasonal influenza vaccine had similar antibody responses compared to individuals who did not receive BIMZELX prior to vaccination. The effectiveness of inactivated seasonal influenza vaccines and other inactivated and non-live vaccines has not been evaluated in patients treated with BIMZELX.
Pharmacokinetics
Bimekizumab-bkzx pharmacokinetics are comparable in adult patients with moderate to severe plaque psoriasis, psoriatic arthritis, non-radiographic axial spondyloarthritis, and ankylosing spondylitis.
The median peak plasma concentration of bimekizumab-bkzx was 25 (range: 12-50) μg/mL and was reached in 3-4 days. Bimekizumab-bkzx exhibited dose-proportional pharmacokinetics in patients with plaque psoriasis over a dose range of 64 mg to 480 mg (0.2 to 1.5 times the approved recommended dosage) following subcutaneous administration.
The median steady-state trough concentration of bimekizumab-bkzx was approximately 40% lower in HS subjects than that of PSO subjects.
Absorption
The absolute bioavailability of bimekizumab-bkzx was 70% in healthy subjects.
Distribution
The median volume of distribution at steady state was 11.2 L in subjects with moderate to severe plaque psoriasis. The volume of distribution in subjects with moderate to severe hidradenitis suppurativa was estimated to be approximately 18% higher than in subjects with moderate to severe plaque psoriasis.
Elimination
The median (coefficient of variation %) clearance (CL/F) of bimekizumab-bkzx was 0.337 L/day (32.7%). The mean terminal elimination half-life was 23 days, with clearance independent of dose. The apparent clearance in subjects with moderate to severe hidradenitis suppurativa was estimated to be approximately 31% higher than in subjects with moderate to severe plaque psoriasis.
Metabolism: Bimekizumab-bkzx is expected to be degraded into small peptides by catabolic pathways.
Specific Populations
No significant differences in the pharmacokinetics of bimekizumab-bkzx were observed based on age (≥18 years).
Body Weight: The average plasma concentration in adult subjects weighing ≥120 kg was predicted to be at least 30% lower than those weighing < 120 kg (median of 87 kg) [see Dosage and Administration (2.2) ].
Immunogenicity
The observed incidence of anti-drug antibodies (ADA) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of ADA in the studies described below with the incidence of ADA in other studies, including those of BIMZELX or of other bimekizumab products.
Across the pivotal trials in all indications, there was no identified clinically significant effect of anti-bimekizumab-bkzx antibodies, including neutralizing anti-drug antibodies, on safety or effectiveness of BIMZELX.
Plaque Psoriasis
During the 52–56-week treatment period in Trial Ps-1, Trial Ps-2, and Trial Ps-3 [see Clinical Studies (14.1) ], 116/257 (45%) of BIMZELX-treated subjects (at the recommended dosage) developed anti-bimekizumab-bkzx antibodies (also referred to as ADA). Of the BIMZELX-treated subjects who developed ADA in these trials, approximately 16% had neutralizing antibodies.
Psoriatic Arthritis
During the 52-week treatment period in Trial PsA-1 [see Clinical Studies (14.2) ] , 201/431 (47%) of subjects treated with BIMZELX had ADA, and 18% had neutralizing ADA.
Non-Radiographic Axial Spondyloarthritis
During the 52-week treatment period in Trial nr-axSpA-1 [see Clinical Studies (14.3) ], 68/119 (57%) of BIMZELX-treated subjects had anti-bimekizumab-bkzx ADA, and approximately 25% had neutralizing ADA.
Ankylosing Spondylitis
During the 52-week treatment period in Trial AS-1 [see Clinical Studies (14.4) ], 86/194 (44%) of BIMZELX-treated subjects had anti-bimekizumab-bkzx ADA, and approximately 20% had neutralizing ADA.
Hidradenitis Suppurativa
During the 48-week treatment period in Trials HS-1 and HS-2 [see Clinical Studies (14.5) ], 171/291 (59%) of BIMZELX-treated subjects had anti-bimekizumab-bkzx ADA, and of those who developed ADA, approximately 63% had neutralizing ADA.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity and mutagenicity studies have not been conducted with bimekizumab-bkzx.
No effects on fertility parameters such as effects on reproductive organs, menstrual cycle length, or sperm analysis were observed in sexually mature cynomolgus monkeys that were subcutaneously administered 200 mg/kg/week bimekizumab-bkzx (150 times the MRHD, based on mg/kg comparison) for 26 weeks. The monkeys were not mated to evaluate fertility.
CLINICAL STUDIES
Plaque Psoriasis
Three multicenter, randomized, double-blind trials [Trial Ps-1 (NCT03370133), Trial Ps-2 (NCT03410992), and Trial Ps-3 (NCT03412747)] enrolled a total of 1,480 subjects 18 years of age and older with moderate to severe plaque psoriasis who had a body surface area (BSA) involvement of ≥10%, an Investigator's Global Assessment (IGA) score of ≥3 ("moderate") in the overall assessment of psoriasis on a severity scale of 0 to 4, and a Psoriasis Area and Severity Index (PASI) score ≥12.
In Trial Ps-1, 567 subjects were randomized to receive either BIMZELX 320 mg by subcutaneous injection every 4 weeks, ustekinumab (for subjects weighing ≤100kg, 45 mg initially and 4 weeks later, then every 12 weeks; for subjects weighing >100kg, 90 mg initially and 4 weeks later, then every 12 weeks), or placebo through Week 52. At Week 16, subjects originally randomized to placebo received BIMZELX 320 mg every 4 weeks through Week 52.
In Trial Ps-2, 435 subjects were randomized to either BIMZELX 320 mg by subcutaneous injection every 4 weeks or placebo. At Week 16, subjects who achieved a PASI 90 response continued into a 40-week randomized withdrawal period. Subjects originally randomized to BIMZELX 320 mg every 4 weeks were re-randomized to either BIMZELX 320 mg every 4 weeks or BIMZELX 320 mg every 8 weeks or placebo. Subjects originally randomized to placebo continued to receive placebo if they were PASI 90 responders. Subjects who did not achieve a PASI 90 response at week 16 entered an open-label escape arm and received BIMZELX 320 mg every 4 weeks for 12 weeks. Subjects who relapsed, defined as having a less than PASI 75 response compared to baseline, during the randomized withdrawal period also entered the 12-week escape arm.
In Trial Ps-3, 478 subjects were randomized to receive either BIMZELX 320 mg by subcutaneous injection every 4 weeks through week 56, BIMZELX 320 mg every 4 weeks through week 16 followed by BIMZELX every 8 weeks through week 56, or adalimumab (80 mg as an initial dose followed by 40 mg every other week starting 1 week after initial dose through Week 24) followed by BIMZELX 320 mg every 4 weeks through Week 56.
In Trial Ps-1, Trial Ps-2, and Trial Ps-3, 71% of the subjects were male and 84% of the subjects were White, with a mean age of 45 years and a mean weight of 89 kg. At baseline, subjects had a median baseline PASI score of 18, median baseline for BSA of 20%, and baseline IGA score of 4 ("severe") in 33% of subjects. A total of 93% subjects had psoriasis of the scalp (Scalp IGA score of ≥1) and a total of 26% of subjects had a history of psoriatic arthritis. Additionally, 38% had received prior biologic therapy.
Clinical Response at Week 16 (Trial Ps-1 and Trial Ps-2)
Trial Ps-1 and Trial Ps-2 responses at Week 16 compared to placebo for the two co-primary endpoints:
- The proportion of subjects who achieved an IGA score of 0 ("clear") or 1 ("almost clear") with at least a 2-grade improvement from baseline
- The proportion of subjects who achieved at least a 90% reduction from baseline PASI (PASI 90)
Secondary endpoints included the proportion of subjects who achieved PASI 100, IGA 0, and Scalp IGA response (defined as Scalp IGA score of 0 [clear] or 1 [almost clear] with at least 2-grade of improvement from baseline) at Week 16, and PASI 75 at Week 4. In addition, secondary endpoints included assessment of psoriasis symptoms (itching, pain, and scaling) measured by the Patient Symptom Diary (PSD) at Week 16.
The proportion of subjects who achieved IGA 0 or 1, PASI 90, IGA 0, and PASI 100 response at Week 16 are presented in Table 3.
| Trial Ps-1 | Trial Ps-2 | |||
|---|---|---|---|---|
| BIMZELX 320 mg every 4 weeks (N=321) n (%) | Placebo (N=83) n (%) | BIMZELX 320 mg every 4 weeks (N=349) n (%) | Placebo (N=86) n (%) | |
| IGA 0 or 1 ("clear" or "almost clear") Co-primary endpoints | 270 (84%) | 4 (5%) | 323 (93%) | 1 (1%) |
| Difference (95% CI) | 79% (73%, 85%) | 91% (88%, 95%) | ||
| PASI 90 | 273 (85%) | 4 (5%) | 317 (91%) | 1 (1%) |
| Difference (95% CI) | 80% (74%, 86%) | 90% (86%, 93%) | ||
| IGA 0 ("clear") | 188 (59%) | 0 (0%) | 243 (70%) | 1 (1%) |
| Difference (95% CI) | 59% (53%, 64%) | 69% (64%, 74%) | ||
| PASI 100 | 188 (59%) | 0 (0%) | 238 (68%) | 1 (1%) |
| Difference (95% CI) | 59% (53%, 64%) | 67% (62%, 73%) | ||
Examination of age, gender, race, baseline IGA score and previous treatment with systemic or biologic agents did not identify differences in response to BIMZELX among these subgroups at Week 16.
A greater proportion of subjects randomized to BIMZELX achieved PASI 75 at Week 4 in both trials compared to placebo. In Trial Ps-1, 77% of subjects treated with BIMZELX achieved PASI 75 compared to 2% treated with placebo. In Trial Ps-2, 76% of subjects treated with BIMZELX achieved PASI 75 compared to 1% treated with placebo.
Among subjects with Scalp IGA score of at least 2 at baseline, a greater proportion of subjects randomized to BIMZELX achieved Scalp IGA response at Week 16 in both trials compared to placebo. In Trial Ps-1, 84% (240/285) of subjects treated with BIMZELX achieved Scalp IGA response compared to 15% (11/72) of placebo treated subjects. In Trial Ps-2, 92% (286/310) of subjects treated with BIMZELX achieved Scalp IGA response compared to 7% (5/74) of placebo treated subjects.
Maintenance and Durability of Response
In Trial Ps-2, subjects randomized to BIMZELX every 4 weeks at Week 0 and who were PASI 90 responders at Week 16 were re-randomized to either continue treatment with BIMZELX every 4 weeks, switched to BIMZELX every 8 weeks, or be withdrawn from therapy (i.e., received placebo).
Figure 1 and Figure 2 present the percentage of subjects maintaining IGA score of 0 ("Clear") or 1 ("Almost Clear") and PASI 90, respectively, through Week 56 after re-randomization at Week 16.
Figure 1: Percentage of Subjects Maintaining IGA 0 or 1 through Week 56 after Re-Randomization at Week 16

For IGA 0 or 1 responders at Week 16 who were re-randomized to treatment withdrawal (i.e., placebo), the median time to loss of IGA 0 or 1 response was approximately 24 weeks. Among these subjects with IGA score of 2 at retreatment, 58% (14/24) achieved IGA score of 0 within 12 weeks of restarting treatment with BIMZELX 320 mg every 4 weeks. Among these subjects with IGA score ≥ 3 at retreatment, 87% (34/39) regained IGA 0 or 1 response with at least 2-grade improvement from retreatment within 12 weeks of restarting treatment with BIMZELX 320 mg every 4 weeks.
Figure 2: Percentage of Subjects Maintaining PASI 90 through Week 56 after Re-Randomization at Week 16

For PASI 90 responders at Week 16 who were re-randomized to treatment withdrawal (i.e., placebo), the median time to loss of PASI 90 response was approximately 24 weeks.
Patient Reported Outcomes
Greater improvements in itch, pain, and scaling at Week 16 with BIMZELX compared to placebo were observed in both trials as measured by the Patient Symptom Diary (PSD).
Psoriatic Arthritis
The safety and efficacy of BIMZELX were assessed in 1,112 subjects in two multicenter, randomized, double-blind, placebo-controlled studies [Trial PsA-1 (NCT 03895203) and Trial PsA-2 (NCT 03896581)] in subjects 18 years and older with active psoriatic arthritis (PsA).
Subjects in these studies had a diagnosis of PsA of at least 6 months based on Classification Criteria for Psoriatic Arthritis (CASPAR), a median duration of 4.6 years at baseline, and active disease with ≥3 tender joint count and ≥3 swollen joint count. Subjects with each subtype of PsA were enrolled in these studies, including polyarticular symmetric arthritis (63.5%), oligoarticular asymmetric arthritis (25.9%), distal interphalangeal joint predominant (4.4%), spondylitis predominant (4.2%), and arthritis mutilans (1.5%). At baseline, 56% of subjects had ≥3% Body Surface Area (BSA) with active plaque psoriasis. At baseline across both studies, 32% and 12% of subjects had enthesitis and dactylitis, respectively, 58% of subjects had psoriatic nail disease, and 53% of subjects were receiving concomitant methotrexate.
The PsA-1 study evaluated 852 biologic-naïve subjects, who were treated with either BIMZELX 160 mg every 4 weeks up to Week 52, adalimumab 40 mg every 2 weeks up to Week 52 (active reference arm), or placebo. Subjects receiving placebo were switched to BIMZELX every 4 weeks at Week 16 to Week 52. In this study, 78% of subjects had received prior treatment with ≥ 1 conventional DMARDs (cDMARDs), and 22 % of subjects had no prior treatment with cDMARDs. At baseline, 58% of subjects were receiving concomitant methotrexate (MTX), 11% were receiving concomitant cDMARDs other than MTX, and 31% were receiving no cDMARDs. The PsA-2 study evaluated 400 anti-TNFα experienced subjects (inadequate response or intolerance to treatment), who were treated with BIMZELX 160 mg every 4 weeks or placebo up to Week 16. In this study, 43% of subjects were receiving concomitant MTX, 8% were receiving concomitant cDMARDs other than MTX, and 50% were receiving no cDMARDs.
For both studies, the primary endpoint was the proportion of subjects who achieved an America College of Rheumatology (ACR) 50 response at Week 16.
Clinical Response
In both studies, treatment with BIMZELX resulted in significant improvement in disease activity, as measured by ACR, compared to placebo at Week 16 (see Table 4 ). Responses in Trial PsA-2 (anti-TNF experienced) were similar to Trial PsA-1.
| Trial PsA-1 – bDMARD naïve | Trial PsA-2 – anti-TNFα experienced | |||||
|---|---|---|---|---|---|---|
| Endpoint | BIMZELX 160 mg Q4W N=431 n (%) | Placebo N=281 n (%) | Difference from Placebo 95% CI based on normal approximation (95% CI) | BIMZELX 160 mg Q4W N=267 n (%) | Placebo N=133 n (%) | Difference from Placebo (95% CI) |
| CI= confidence interval | ||||||
| ACR 20 Response | ||||||
| Week 16 | 268 (62.2) | 67 (23.8) | 38.3 (31.6, 45.1) | 179 (67.0) | 21 (15.8) | 51.3 (42.9, 59.6) |
| ACR 50 Response | ||||||
| Week 16 | 189 (43.9) Multiplicity-controlled p<0.001 | 28 (10.0) | 33.9 (28.0, 39.7) | 116 (43.4) | 9 (6.8) | 36.7 (29.4, 44.0) |
| ACR 70 Response | ||||||
| Week 16 | 105 (24.4) | 12 (4.3) | 20.1 (15.4, 24.8) | 71 (26.6) | 1 (0.8) | 25.8 (15.6, 35.7) Exact 95% CI used |
The percentage of subjects achieving ACR50 responses in Trial PsA-1 by visit through Week 16 is shown in Figure 3. Similar responses were seen in Trial PsA-2 up to Week 16.
Figure 3: Percent of Subjects Achieving ACR 50 Responses in Trial PsA-1 through Week 16

The results of the components of the ACR response criteria are shown in Table 5.
| Trial PsA-1 – bDMARD naïve | Trial PsA-2 – anti-TNFα experienced | |||
|---|---|---|---|---|
| Placebo (N=281) | BIMZELX 160 mg Q4W (N=431) | Placebo (N=133) | BIMZELX 160 mg Q4W (N=267) | |
| Multiple Imputation (MI) is used for all endpoints presented in Table 5. | ||||
| Number of Swollen Joints | ||||
| Baseline | 9.5 | 9.0 | 10.3 | 9.7 |
| Mean change at Week 16 | -3.0 | -6.6 | -2.0 | -7.0 |
| Number of Tender Joints | ||||
| Baseline | 17.1 | 16.8 | 19.3 | 18.4 |
| Mean change at Week 16 | -3.2 | -10.0 | -2.4 | -10.9 |
| Patient's Assessment of Pain | ||||
| Baseline | 56.8 | 53.7 | 61.7 | 58.3 |
| Mean change at Week 16 | -6.2 | -23.6 | -4.5 | -27.7 |
| Patient's Global Assessment | ||||
| Baseline | 58.6 | 54.4 | 63.0 | 60.5 |
| Mean change at Week 16 | -7.7 | -26.3 | -5.5 | -31.8 |
| Physician Global Assessment | ||||
| Baseline | 57.3 | 57.2 | 57.7 | 59.3 |
| Mean change at Week 16 | -12.5 | -37.4 | -6.8 | -49.4 |
| Health Assessment Questionnaire- Disability Index (HAQ-DI) | ||||
| Baseline | 0.9 | 0.8 | 1.0 | 1.0 |
| Mean Change at Week 16 | -0.1 | -0.3 p<0.001 reference-based imputation versus placebo adjusted for multiplicity. | -0.1 | -0.4 |
| High Sensitivity C-reactive Protein (hsCRP) mg/L | ||||
| Baseline | 11.4 | 8.7 | 11.6 | 12.4 |
| Mean Change at Week 16 | -2.4 | -4.2 | 3.6 | -7.0 |
Treatment with BIMZELX resulted in improvement in dactylitis and enthesitis in subjects with pre-existing dactylitis or enthesitis, compared to placebo.
In subjects with coexistent plaque psoriasis receiving BIMZELX, the skin lesions of psoriasis improved with treatment, relative to placebo, as measured by the Psoriasis Area Severity Index (PASI 90) at Week 16.
Radiographic Response
In Trial PsA-1, inhibition of progression of structural damage was assessed radiographically and expressed as the change from baseline in the Van der Heijde modified total Sharp Score (vdHmTSS) and its components, the Erosion Score (ES) and the Joint Space Narrowing score (JSN), at Week 16 (see Table 6 ).
BIMZELX significantly inhibited the rate of progression of joint damage at Week 16 in the overall population compared to placebo. The change from Baseline in erosion subscores contributed more to the change from Baseline in vdHmTSS total score than the change from Baseline in joint narrowing subscore. The percentage of subjects with no radiographic joint damage progression (defined as a change from baseline in mTSS of ≤0.0) from randomization to Week 16 was 77% for BIMZELX and 69% for placebo in the overall population. Similar responses were achieved in the population with elevated hsCRP and/or at least 1 bone erosion (75% for BIMZELX and 67% for placebo).
| Placebo | BIMZELX 160 mg Q4W | Difference from Placebo (95% CI) Unadjusted differences are shown | |
|---|---|---|---|
| Overall population | (N=269) | (N=420) | |
| Baseline mean (SE) | 12.34 (1.37) | 12.47 (1.46) | |
| Mean change from baseline at Week 16 (SE) | 0.32 (0.09) | 0.04 (0.04) p≤0.001 versus placebo. p-values are based on reference-based imputation using difference in LS Mean using an ANCOVA model with treatment, bone erosion at Baseline and region as fixed effects and Baseline score as a covariate. | -0.26 (-0.29, -0.23) |
Physical Function
In both studies, subjects treated with BIMZELX showed statistically significant improvement from baseline in physical function compared with placebo as assessed by HAQ-DI at Week 16 (see Table 5 ). In both studies, a greater proportion of subjects achieved a reduction of at least 0.35 in HAQ-DI score from baseline in the BIMZELX group compared with placebo at Week 16.
Other Health Related Outcomes
Fatigue was assessed by Functional Assessment of Chronic Illness Therapy Fatigue Scale (FACIT-Fatigue). Additionally, in both studies at Week 16, subjects treated with BIMZELX showed improvements in FACIT-Fatigue scores.
Non-Radiographic Axial Spondyloarthritis
The efficacy and safety were assessed in 254 patients in one randomized, double-blind, placebo-controlled study [Trial nr-axSpA-1 (NCT03928704)] in adult subjects 18 years of age and older with active non-radiographic axial spondyloarthritis. Subjects had to have objective signs of inflammation with elevated C-reactive protein (CRP) level and/or evidence of sacroiliitis on Magnet Resonance Imaging (MRI). Subjects met ASAS classification criteria for axial spondyloarthritis and have active disease as defined by BASDAI greater than or equal to 4, spinal pain of greater than or equal to 4 (0-10 numeric rating scale (NRS)), and no definitive radiographic evidence of structural damage in the sacroiliac joints. At baseline, 73% of subjects had enthesitis. Subjects also had a history of inadequate response to 2 different non-steroidal anti-inflammatory drugs (NSAIDs), or intolerance or contraindication to NSAIDs. Approximately 24% of subjects were on concomitant cDMARDs. Overall, 11% of subjects had received previous treatment (failed or were intolerant to) with anti-TNF alpha agents.
Subjects were randomized to receive BIMZELX 160 mg or placebo every 4 weeks up to the completion of Week 16 assessments. Starting at Week 16, all subjects received BIMZELX every 4 weeks up to Week 52. The primary endpoint was at least 40% improvement in Assessment of Spondyloarthritis International Society (ASAS 40) at Week 16.
Clinical Response
In Trial nr-axSpA-1, treatment with BIMZELX resulted in significant improvements in the measure of disease activity compared to placebo at Week 16 (Table 7).
| BIMZELX 160 mg Q4W (N=128) | Placebo (N=126) | Difference from placebo (95% CI) 95% CI based on normal approximation | |
|---|---|---|---|
| n (%) | n (%) | ||
| NRI is used CI= confidence interval | |||
| ASAS 40 response | 61 (47.7%) Multiplicity-controlled p<0.001 | 27 (21.4%) | 26.2 (15.0, 37.5) |
| ASAS 20 response | 88 (68.8%) | 48 (38.1%) | 30.7 (19.0, 42.3) |
Similar responses were seen regardless of prior anti-TNF alpha therapy. Treatment with BIMZELX resulted in improvement in enthesitis in subjects with pre-existing enthesitis.
The results of the main components of the ASAS 40 response criteria and other measures of disease activity are shown in Table 8.
| BIMZELX 160 mg Q4W (N= 128) | Placebo (N=126) | |
|---|---|---|
| BASFI = Bath Ankylosing Spondylitis Functional Index | ||
| BASMI = Bath Ankylosing Spondylitis Metrology Index | ||
| BASDAI = Bath Ankylosing Spondylitis Disease Activity Index | ||
| MI is used for all endpoints presented in Table 8 | ||
| ASAS Components | ||
| ||
| Baseline | 7.1 | 6.9 |
| Mean Change from Baseline | -3.2 | -1.4 |
| ||
| Baseline | 7.3 | 7.1 |
| Mean Change from Baseline | -3.4 | -1.7 |
| ||
| Baseline | 5.5 | -2.5 Multiplicity-controlled p<0.001 |
| Mean Change from Baseline | 5.3 | -1.0 |
| ||
| Baseline | 7.0 | 6.9 |
| Mean Change from Baseline | -3.6 | -1.9 |
| Other Measures of Disease Activity | ||
| BASDAI Score | ||
| Baseline | 6.9 | 6.7 |
| Mean Change from Baseline | -3.1 | -1.5 |
| BASMI | ||
| Baseline | 2.9 | 3.0 |
| Mean Change from Baseline | -0.4 | -0.1 |
| hsCRP (mg/L) | ||
| Baseline | 11.1 | 10.2 |
| Mean Change from Baseline | -6.7 | 0.0 |
The percentage of subjects achieving ASAS 40 responses in Trial nr-axSpA-1 by visit through Week 16 is shown in Figure 4.
Figure 4: Percent of Subjects Achieving ASAS 40 Responses in Trial nr-axSpA-1 Week 16

Health Related Quality of Life
BIMZELX treated subjects showed significantly greater improvement compared to subjects receiving placebo at Week 16 in health-related quality of life as measured by the Ankylosing Spondylitis Quality of Life Questionnaire (ASQoL) score.
Ankylosing Spondylitis
The efficacy and safety were assessed in 332 patients in one randomized, double-blind, placebo-controlled study [Trial AS-1 (NCT03928743)] in adult subjects 18 years of age and older with active ankylosing spondylitis. Subjects had to have documented radiologic evidence (x-ray) fulfilling the Modified New York criteria for AS. Subjects had active disease as defined by BASDAI ≥4 and spinal pain ≥4 on a 0 to 10 numeric rating scale (NRS) (from BASDAI Item 2). Subjects also had a history of inadequate response to 2 different non-steroidal anti-inflammatory drugs (NSAIDs), or intolerance or contraindication to NSAIDs. Approximately 20% of subjects were on concomitant cDMARDs. Overall, 16% of subjects had received previous treatment (failed or were intolerant to) with anti-TNF alpha agents.
Subjects were randomized 2:1 to receive BIMZELX 160 mg or placebo every 4 weeks up to the completion of Week 16 assessments. Starting at Week 16, all subjects received BIMZELX every 4 weeks up to Week 52. The primary endpoint was at least 40% improvement in Assessment of Spondyloarthritis International Society (ASAS 40) at Week 16.
Clinical Response
In Trial AS-1, treatment with BIMZELX resulted in significant improvements in the measure of disease activity compared to placebo at Week 16 (Table 9).
| BIMZELX 160 mg Q4W (N=221) | Placebo (N=111) | Difference from placebo (95% CI) 95% CI based on normal approximation | |
|---|---|---|---|
| n (%) | n (%) | ||
| NRI is used CI= confidence interval | |||
| ASAS 40 response | 99 (44.8%) Multiplicity-controlled p<0.001 | 25 (22.5%) | 22.3 (12.1, 32.4) |
| ASAS 20 response | 146 (66.1%) | 48 (43.2%) | 22.8 (11.7, 34.0) |
Similar responses were seen regardless of prior anti-TNF alpha therapy. Treatment with BIMZELX resulted in improvement in enthesitis in subjects with pre-existing enthesitis.
The results of the main components of the ASAS 40 response criteria and other measures of disease activity are shown in Table 10.
| BIMZELX 160 mg Q4W (N= 221) | Placebo (N=111) | |
|---|---|---|
| BASFI = Bath Ankylosing Spondylitis Functional Index | ||
| BASMI = Bath Ankylosing Spondylitis Metrology Index | ||
| BASDAI = Bath Ankylosing Spondylitis Disease Activity Index | ||
| MI is used for all endpoints presented in Table 10 | ||
| ASAS Components | ||
| ||
| Baseline | 6.6 | 6.7 |
| Mean Change from Baseline | -2.7 | -1.6 |
| ||
| Baseline | 7.1 | 7.2 |
| Mean Change from Baseline | -3.3 | -1.9 |
| ||
| Baseline | 5.3 | 5.2 |
| Mean Change from Baseline | -2.2 Multiplicity-controlled p<0.001 | -1.1 |
| ||
| Baseline | 6.7 | 6.8 |
| Mean Change from Baseline | -3.2 | -2.1 |
| Other Measures of Disease Activity | ||
| BASDAI Score | ||
| Baseline | 6.4 | 6.5 |
| Mean Change from Baseline | -2.9 | -1.9 |
| BASMI | ||
| Baseline | 3.9 | 3.8 |
| Mean Change from Baseline | -0.5 Multiplicity-controlled p<0.006 | -0.2 |
| hsCRP (mg/L) | ||
| Baseline | 14.7 | 13.6 |
| Mean Change from Baseline | -8.6 | -2.2 |
The percentage of subjects achieving ASAS 40 responses in Trial AS-1 by visit through Week 16 is shown in Figure 5.
Figure 5: Percent of Subjects Achieving ASAS 40 Responses in Trial AS-1 Through Week 16

Health Related Quality of Life
BIMZELX treated subjects showed significantly greater improvement compared to subjects receiving placebo at Week 16 in health-related quality of life as measured by the Ankylosing Spondylitis Quality of Life Questionnaire (ASQoL) score.
Hidradenitis Suppurativa
The safety and efficacy of BIMZELX were assessed in two Phase 3 multicenter, randomized, double-blind, placebo-controlled trials [Trial HS-1 (NCT04242446) and Trial HS-2 (NCT04242498)] in 1,014 adult subjects with moderate to severe HS of at least 6 months with Hurley Stage II or Hurley Stage III disease, and with ≥5 inflammatory lesions [i.e., number of abscesses plus number of inflammatory nodules (AN count)], and a history of inadequate response to a course of systemic antibiotics for the treatment of HS.
Subjects received BIMZELX 320 mg every 2 weeks (Q2W) for 48 weeks, or BIMZELX 320 mg every 4 weeks (Q4W) up to Week 48, or BIMZELX 320 mg Q2W to Week 16, followed by 320 mg Q4W up to Week 48, or placebo. At Week 16, subjects receiving placebo were switched to BIMZELX 320 mg Q2W to Week 48. Concomitant oral doxycycline, minocycline, or an equivalent systemic tetracycline for HS was allowed if the subject was on a stable dose regimen for 28 days prior to baseline.
In these trials, at baseline the mean age of all subjects was 37 years, 57% of subjects were female, 80% were White, 11% were Black or African American, and 4% were Asian; for ethnicity, 7% identified as Hispanic or Latino. Of the subjects enrolled in trials conducted in the United States, 33% were Black or African American. The mean BMI was 33, and 46% were current smokers. Subjects had a median disease duration of 5 years. Overall, 9% of subjects were receiving concomitant antibiotic therapy for HS, and 19% of subjects had received previous treatment with biologics. The proportion of Hurley Stage II and Stage III subjects were 56% and 44%, respectively. Additionally, the mean number of draining tunnels was 3.6 and mean AN count was 16.3.
The primary efficacy endpoint in both trials was the Hidradenitis Suppurativa Clinical Response 50 (HiSCR50) at Week 16, defined by at least a 50% reduction in total abscess and inflammatory nodule count with no increase in abscess or draining tunnel count relative to baseline. Secondary endpoints included the proportion of subjects who achieved HiSCR75 and HS-specific skin pain response as assessed by a 0 to 10 numeric rating scale (NRS).
Clinical Response at Week 16 (Trials HS-1 and HS-2)
In both trials, a higher proportion of BIMZELX-treated subjects achieved HiSCR50 and HiSCR75 compared to placebo (see Table 11 ).
| Trial HS-1 | Trial HS-2 | |||
|---|---|---|---|---|
| BIMZELX 320mg Q2W (N=289) | Placebo (N=72) | BIMZELX 320 mg Q2W (N=291) | Placebo (N=74) | |
| HiSCR50 | 48% | 29% | 52% | 32% |
| Difference (95% CI) | 18% (6%, 30%) | 20% (8%, 32%) | ||
| HiSCR75 | 33% | 18% | 36% | 16% |
| Difference (95% CI) | 15% (4%, 27%) | 20% (10%, 31%) | ||
Examination of age, gender, race, disease duration, weight, prior biologic therapy, systemic antibiotic use at baseline, Hurley stage, and smoking status did not identify meaningful differences in response to BIMZELX among these subgroups at Week 16.
Figure 6 presents the proportion of subjects achieving HiSCR50 response in Trials HS-1 and HS-2 by visit through Week 16.
Figure 6: HiSCR50 Response through Week 16 in Adults with HS in Trials HS-1 and HS-2

Subjects who initiated systemic antibiotics (new antibiotic or change in the dose/type of current antibiotic) for any reason through Week 16 or who discontinued due to adverse event or lack of efficacy are treated as non-responders at all subsequent visits. Other missing data were imputed via multiple imputation.
BIMZELX was associated with an improvement in patient-reported worst skin pain (lesion pain) based on the achievement of a reduction of at least 3 points (as measured on a 0 to 10 NRS) compared to placebo at Week 16.
HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
BIMZELX (bimekizumab-bkzx) injection is a sterile, preservative-free, clear to slightly opalescent, and colorless to pale brownish-yellow solution. Each prefilled autoinjector or prefilled syringe contains 1 mL or 2 mL of a 160 mg/mL solution. The autoinjectors and prefilled syringes are not made with natural rubber latex. BIMZELX is supplied as:
BIMZELX autoinjector:
- NDC 50474-781-85: Carton of two 160 mg/mL single-dose autoinjectors. Each prefilled autoinjector is fixed with a 27 gauge ½ inch needle.
- NDC 50474-781-84: Carton of one 160 mg/mL single-dose autoinjector. The prefilled autoinjector is fixed with a 27 gauge ½ inch needle.
- NDC 50474-782-84: Carton of one 320 mg/2 mL (160 mg/mL) single-dose autoinjector fixed with a 27 gauge ½ inch needle.
BIMZELX prefilled syringe:
- NDC 50474-780-79: Carton of two 160 mg/mL single-dose prefilled syringes. Each prefilled syringe is fixed with a 27 gauge ½ inch needle with needle guard.
- NDC 50474-780-78: Carton of one 160 mg/mL single dose prefilled syringe. The prefilled syringe is fixed with a 27 gauge ½ inch needle with a needle guard.
- NDC 50474-783-78: Carton of one 320 mg/2 mL (160 mg/mL) single-dose prefilled syringe with a 27 gauge ½ inch needle.
Storage and Handling
Store cartons with BIMZELX refrigerated between 2°C to 8°C (36°F to 46°F). Keep the product in the original carton to protect it from light until the time of use. Do not freeze. Do not shake. Do not use beyond expiration date. BIMZELX does not contain a preservative; discard any unused portion.
When necessary, BIMZELX prefilled syringes or autoinjectors may be stored at room temperature up to 25°C (77°F) in the original carton for a single period of up to 30 days. Once BIMZELX prefilled syringes or autoinjectors have been stored at room temperature, do not place back in refrigerator. Write the date removed from the refrigerator in the space provided on the carton and discard if not used within a 30-day period.
INSTRUCTIONS for USE BIMZELX ® (bim zel' ex) (bimekizumab-bkzx) injection, for subcutaneous use single-dose autoinjector
This Instructions for Use contains information on how to inject BIMZELX.
Read this Instructions for Use before using the BIMZELX autoinjector and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
These instructions are for 1 injection only. You may need more than 1 injection at a time depending on your prescribed dose of BIMZELX. Each BIMZELX autoinjector is for one-time (single-dose) use only.
Important Information you Need to Know Before Injecting BIMZELX:
- Your healthcare provider should show you or a caregiver how to prepare and inject BIMZELX using the autoinjector for the first time. Do not inject yourself or someone else until you have been shown how to inject BIMZELX the right way. Call your healthcare provider if you have any questions.
- These instructions are for 1 injection only.
- The BIMZELX autoinjector has a needle safety feature that will be activated to cover the needle after the injection is finished. The needle safety feature will help to prevent needle stick injuries to anyone who handles the autoinjector after injection.
- Do not share or reuse your BIMZELX autoinjector. You may give or get an infection.
- Do not remove the needle cap until just before you give the injection.
- If you have vision or hearing problems, do not use the BIMZELX autoinjector without help from a caregiver.
How should I store BIMZELX autoinjector?
- Store the BIMZELX autoinjector in the refrigerator between 36°F to 46°F (2°C to 8°C).
- BIMZELX autoinjectors may be stored at room temperature up to 77°F (25°C) in the original carton for up to 30 days. Do not place BIMZELX autoinjectors back in the refrigerator after they have been stored at room temperature.
- Write the date removed from the refrigerator in the space provided on the carton and throw away if it has been kept at room temperature and not used within 30 days.
- Keep BIMZELX in the original carton until ready for use to protect from light.
- Do not freeze BIMZELX.
- Do not shake BIMZELX.
- Do not use the BIMZELX autoinjector past the expiration date printed on the carton.
Keep BIMZELX autoinjectors and all medicines out of the reach of children.
BIMZELX autoinjector parts (see Figure A ):
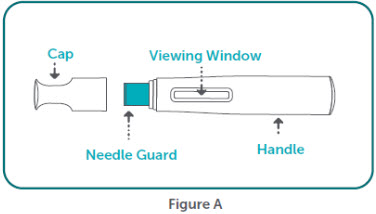
Supplies Needed for each BIMZELX injection
- 1 BIMZELX autoinjector. You may need 2 BIMZELX autoinjectors to give the prescribed dose.
Not provided in the BIMZELX autoinjector carton:
- 1 alcohol swab
- 1 clean cotton ball
- 1 sharps disposal container. See, " Step 12: Dispose of (throw away) the used BIMZELX autoinjector " at the end of this Instructions for Use.
Setting up for your BIMZELX injection.
Step 1: Take the BIMZELX autoinjector carton out of the refrigerator. Do not use the BIMZELX autoinjector(s) if the carton seal is broken. Throw it away and get a new one.
- Keep the BIMZELX autoinjector in its original carton for 30 to 45 minutes to warm to room temperature. This will help to reduce discomfort when injecting.
- Do not microwave the autoinjector, run hot water over it, or leave it in direct sunlight.
- Do not shake the autoinjector.
- Do not take the cap off the autoinjector until you are ready to inject.
Step 2 : Find a clean, flat, and well-lit work surface, like a table.
Step 3: Gather the supplies needed for your injection.
Step 4: Wash your hands well with soap and water and dry with a clean towel.
Step 5: Inspect the BIMZELX autoinjector (see Figure B ):
- Remove the BIMZELX autoinjector from the carton.
- Make sure the name BIMZELX appears on the label.
- Check the expiration date printed on the label.
- Check the medicine through the Viewing Window. The medicine inside should be clear to slightly pearly, and colorless to pale brownish-yellow. You may see air bubble(s) in the liquid. This is normal.
- Do not use the BIMZELX autoinjector, throw it away and get a new one if:
- The expiration date printed on the label has passed.
- The medicine is cloudy, discolored, or has particles.
- It looks damaged or has been dropped.
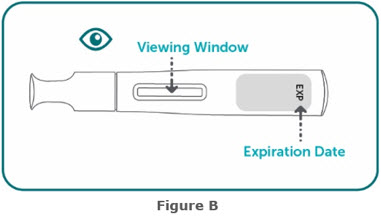
Choose and prepare your injection site.
Step 6: Choose and clean your injection site.
- The sites you may choose for your injection are :
|
|
- Do not inject into areas where the skin is tender, bruised, red, hard, thick, scaly, or affected by psoriasis , or within 2 inches of the belly button (navel).
- Choose a new injection site on the body each time you use BIMZELX. Do not use the same injection site 2 times in a row.
Step 7: Prepare your skin
- Clean the injection site with an alcohol swab. Let the area dry completely. Do not touch the cleaned area again before injecting.
Injecting BIMZELX.
Step 8: Remove the BIMZELX autoinjector cap.
- Hold the BIMZELX autoinjector firmly with one hand around the handle. Pull the cap straight off (away from) the autoinjector with the other hand ( see Figure E ). Although you cannot see the needle tip, it is now uncovered.
- Put the cap into an FDA-cleared sharps disposal container right away. You will not need it again.
- Do not touch the needle guard or put the cap back on as it could activate the autoinjector and you can stick yourself.
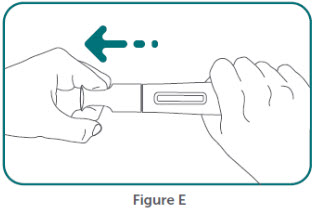
Step 9: Hold the BIMZELX autoinjector at a 90-degree angle to the cleaned injection site (see Figure F ).
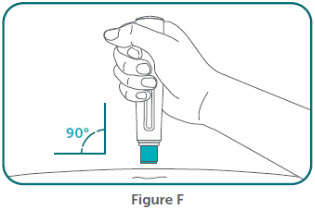
Step 10: Place the BIMZELX autoinjector flat against your skin, then firmly press the BIMZELX autoinjector down against your skin. You will hear a "click" sound . Your injection begins when the 1 st "click" is heard (see Figure G ) .
- Do not lift the autoinjector away from the skin.
Keep holding the BIMZELX autoinjector in place and pressed firmly against your skin. It will take about 15 seconds to receive your full dose.
- You will hear a 2 nd "click" in about 15 seconds after you hear the first click.
- The second click tells you that all the medicine has been injected and your BIMZELX injection is finished. You should see the yellow color indicator filling the viewing window (see Figure H ) .
- Important: When you remove the autoinjector, if the viewing window has not turned yellow this means you may not have received a full dose. Call your healthcare provider right away.
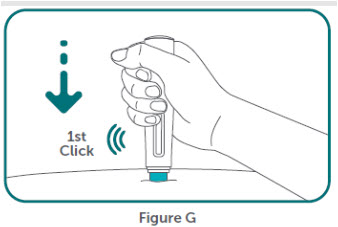
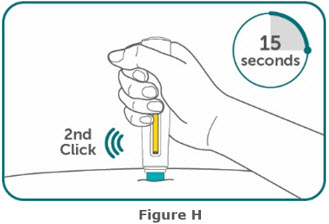
Step 11: Remove the BIMZELX autoinjector by carefully pulling the BIMZELX autoinjector straight up from your skin. The needle guard will automatically cover the needle. Do not try to touch the needle.
- Press a clean cotton ball over the injection site for a few seconds. Do not rub the injection site. You may see slight bleeding or a drop of liquid. This is normal. You may cover the injection site with a small adhesive bandage, if needed.
Step 12: Dispose of (throw away) the used BIMZELX autoinjector (see Figure I ).
- Put the used BIMZELX autoinjector in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) the BIMZELX autoinjector in your household trash. If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal .
- Do not recycle your used sharps disposal container.
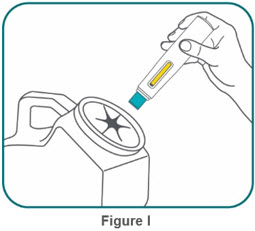
Step 13: Repeat steps 1 through 12 with a new BIMZELX autoinjector if you need to use 2 autoinjectors to give the prescribed dose.
- Make sure you select a new injection site on the body each time you use BIMZELX. Do not use the same site that you used for your first injection.
You can sign up to receive sharps containers for BIMZELX autoinjector disposal at no additional cost by going to www.BIMZELX.com or call 1-844-599-2273.
Manufactured by: UCB, Inc. 1950 Lake Park Drive Smyrna, GA 30080 US License No. 1736
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Issued: 9/2024
Mechanism of Action
Bimekizumab-bkzx is a humanized immunoglobulin IgG1/ κ monoclonal antibody with two identical antigen binding regions that selectively bind to human interleukin 17A (IL-17A), interleukin 17F (IL-17F), and interleukin 17-AF cytokines, and inhibits their interaction with the IL-17 receptor complex. IL-17A and IL-17F are naturally occurring cytokines that are involved in normal inflammatory and immune responses. Bimekizumab-bkzx inhibits the release of proinflammatory cytokines and chemokines.