Cetirizine Hydrochloride Oral Solution
Cetirizine Hydrochloride Oral Solution Prescribing Information
Cetirizine Hydrochloride Oral Solution, USP can be taken without regard to food consumption.
Cetirizine Hydrochloride Oral Solution, USP is contraindicated in those patients with a known hypersensitivity to it or any of its ingredients or hydroxyzine.
Pediatric studies were conducted with cetirizine hydrochloride. More than 1300 pediatric patients aged 6 to 11 years with more than 900 treated with cetirizine hydrochloride at doses of 1.25 to 10 mg per day were included in controlled and uncontrolled clinical trials conducted in the United States. The duration of treatment ranged from 2 to 12 weeks. Placebo-controlled trials up to 4 weeks duration included 168 pediatric patients aged 2 to 5 years who received cetirizine, the majority of whom received single daily doses of 5 mg. A placebo-controlled trial 18 months in duration included 399 patients aged 12 to 24 months treated with cetirizine (0.25 mg/kg bid), and another placebo-controlled trial of 7 days duration included 42 patients aged 6 to 11 months who were treated with cetirizine (0.25 mg/kg bid).
The majority of adverse reactions reported in pediatric patients aged 2 to 11 years with cetirizine hydrochloride were mild or moderate. In placebo-controlled trials, the incidence of discontinuations due to adverse reactions in pediatric patients receiving up to 10 mg of cetirizine hydrochloride was uncommon (0.4% on cetirizine hydrochloride vs. 1.0% on placebo).
Table 1 lists adverse experiences which were reported for cetirizine hydrochloride 5 and 10 mg in pediatric patients aged 6 to 11 years in placebo-controlled clinical trials in the United States and were more common with cetirizine hydrochloride than placebo. Of these, abdominal pain was considered treatment-related and somnolence appeared to be dose-related, 1.3% in placebo, 1.9% at 5 mg and 4.2% at 10 mg. The adverse experiences reported in pediatric patients aged 2 to 5 years in placebo-controlled trials were qualitatively similar in nature and generally similar in frequency to those reported in trials with children aged 6 to 11 years.
In the placebo-controlled trials of pediatric patients 6 to 24 months of age, the incidences of adverse experiences were similar in the cetirizine and placebo treatment groups in each study. Somnolence occurred with essentially the same frequency in patients who received cetirizine and patients who received placebo. In a study of 1 week duration in children 6-11 months of age, patients who received cetirizine exhibited greater irritability/fussiness than patients on placebo. In a study of 18 months duration in patients 12 months and older, insomnia occurred more frequently in patients who received cetirizine compared to patients who received placebo (9.0% v. 5.3%). In those patients who received 5 mg or more per day of cetirizine as compared to patients who received placebo, fatigue (3.6% v. 1.3%) and malaise (3.6% v. 1.8%) occurred more frequently.
Adverse Experiences | Placebo (N=309) | Cetirizine Hydrochloride | |
5 mg (N=161) | 10mg (N=215) | ||
| Headache | 12.3% | 11.0% | 14.0% |
| Pharyngitis | 2.9% | 6.2% | 2.8% |
| Abdominal Pains | 1.9% | 4.4% | 5.6% |
| Coughing | 3.9% | 4.4% | 2.8% |
| Somnolence | 1.3% | 1.9% | 4.2% |
| Diarrhea | 1.3% | 3.1% | 1.9% |
| Epistaxis | 2.9% | 3.7% | 1.9% |
| Bronchospasm | 1.9% | 3.1% | 1.9% |
| Nausea | 1.9% | 1.9% | 2.8% |
| Vomiting | 1.0% | 2.5% | 2.3% |
The following events were observed infrequently (less than 2%), in either 3,982 adults and children 12 years and older or in 659 pediatric patients aged 6 to 11 years who received cetirizine hydrochloride in U.S. trials, including an open adult study of six months duration. A causal relationship of these infrequent events with cetirizine hydrochloride administration has not been established.
Occasional instances of transient, reversible hepatic transaminase elevations have occurred during cetirizine therapy. Hepatitis with significant transaminase elevation and elevated bilirubin in association with the use of cetirizine hydrochloride has been reported.
In the post-marketing period, the following additional rare, but potentially severe adverse events have been reported: aggressive reaction, anaphylaxis, cholestasis, convulsions, glomerulonephritis, hallucinations, hemolytic anemia, hepatitis, orofacial dyskinesia, severe hypotension, stillbirth, suicidal ideation, suicide, thrombocytopenia, acute generalized exanthematous pustulosis (AGEP) and rebound pruritus-pruritus within a few days after discontinuation of cetirizine, usually after long-term use (e.g., months to years) of cetirizine.
To report SUSPECTED ADVERSE REACTIONS, contact Apozeal Pharmaceuticals Inc. at tel: 1-833-688-7848 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
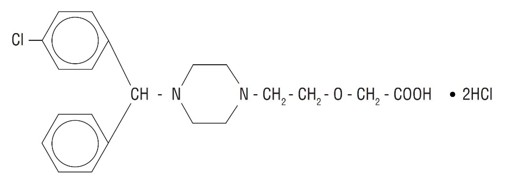
Cetirizine hydrochloride is an orally active and selective HI-receptor antagonist. The chemical name is(±) - [2- [4- [(4-chlorophenyl)phenylmethyl]-1- piperazinyl] ethoxy] acetic acid, dihydrochloride. Cetirizine hydrochloride is a racemic compound with an empirical formula of C21H25ClN203 •2HCL The molecular weight is 461.82 and the chemical structure is shown below:
Cetirizine hydrochloride, USP is a white or almost white powder and is freely soluble in water, practically insoluble in acetone and in methylene chloride. Cetirizine Hydrochloride Oral Solution, USP is a colorless to slightly yellow oral solution containing cetirizine hydrochloride at a concentration of 1 mg/mL (5 mg/5 mL) for oral administration. The pH is between 4 and 5. The inactive ingredients of the oral solution are: glacial acetic acid, grape flavor, glycerin, methylparaben, propylene glycol, propylparaben, sodium acetate, sucrose and purified water.
Pharmacokinetic interaction studies with cetirizine in adults were conducted with pseudoephedrine, antipyrine, ketoconazole, erythromycin and azithromycin.
No interactions were observed. In a multiple dose study oftheophylline (400 mg once daily for 3 days) and cetirizine (20 mg once daily for 3 days), a 16% decrease in the clearance of cetirizine was observed. The disposition of theophylline was not altered by concomitant cetirizine administration.
Pediatric Patients: In pediatric patients aged 2 to 5 years who received 5 mg of cetirizine, the mean Cmax was 660 ng/mL. Based on cross study comparisons, the weight normalized, apparent total body clearance was 81-111 % greater and the elimination half life was 33 to 41 % shorter in the pediatric population than in adults. In pediatric patients aged 6 to 23 months who received a single dose of 0.25 mg/kg cetirizine oral solution (mean dose 2.3 mg), the mean Cmax was 390 ng/mL. Based on cross-study comparisons, the weight-normalized, apparent total body clearance was 304% greater and the elimination half-life was 63% shorter in this pediatric population compared to adults. The average AUC (O-t) in children 6 months to < 2 years of age receiving the maximum dose of cetirizine solution (2.5 mg twice a day) is expected to be two-fold higher than that observed in adults receiving a dose of 10 mg cetirizine tablets once a day.
Effect of Gender: The effect of gender on cetirizine pharmacokinetics has not been adequately studied.
Effect of Race: No race-related differences in the kinetics of cetirizine have been observed.
Pharmacodynamics: Cetirizine hydrochloride at doses of 5 and 10 mg strongly inhibited the wheal and flare caused by intradermal injection of histamine in 19 pediatric volunteers (aged 5 to 12 years) and the activity persisted for at least 24 hours. In a 35 day study in children aged 5 to 12, no tolerance to the antihistaminic (suppression of wheal and flare response) effects of cetirizine hydrochloride was found. In 10 infants 7 to 25 months of age who received 4 to 9 days of cetirizine in an oral solution (0.25 mg/kg bid), there was a 90% inhibition of histamine-induced (10 mg/mL) cutaneous wheal and 87% inhibition of the flare 12 hours after administration of the last dose. The clinical relevance of this suppression of histamine-induced wheal and flare response on skin testing is unknown.
The effects of intradermal injection of various other mediators or histamine releasers were also inhibited by cetirizine, as was response to a cold challenge in patients with cold-induced urticaria. In mildly asthmatic subjects, cetirizine hydrochloride at 5 to 20 mg blocked bronchoconstriction due to nebulized histamine, with virtually total blockade after a 20 mg dose. In studies conducted for up to 12 hours following cutaneous antigen challenge, the late phase recruitment of eosinophils, neutrophils and basophils, components of the allergic inflammatory response, was inhibited by cetirizine hydrochloride at a dose of 20 mg. In four clinical studies in healthy adult males, no clinically significant mean increases in QTc were observed in cetirizine hydrochloride treated subjects. In the first study, a placebo-controlled crossover trial, cetirizine hydrochloride was given at doses up to 60 mg per day, 6 times the maximum clinical dose, for 1 week, and no significant mean QTc prolongation occurred. In the second study, a crossover trial, cetirizine hydrochloride 20 mg and erythromycin (500 mg every 8 hours) were given alone and in combination. There was no significant effect on QTc with the combination or with cetirizine hydrochloride alone. In the third trial, also a crossover study, cetirizine hydrochloride 20 mg and ketoconazole (400 mg per day) were given alone and in combination. Cetirizine hydrochloride caused a mean increase in QTc of 9.1 msec from baseline after 10 days of therapy. Ketoconazole also increased QTc by 8.3 msec. The combination caused an increase of 17.4 msec, equal to the sum of the individual effects. Thus, there was no significant drug interaction on QTc with the combination of cetirizine hydrochloride and ketoconazole. In the fourth study, a placebo-controlled parallel trial, cetirizine hydrochloride 20 mg was given alone or in combination with azithromycin (500 mg as a single dose on the first day followed by 250 mg once daily). There was no significant increase in QTc with cetirizine hydrochloride 20 mg alone or in combination with azithromycin. In a four-week clinical trial in pediatric patients aged 6 to 11 years, results of randomly obtained ECG measurements before treatment and after 2 weeks of treatment showed that cetirizine hydrochloride 5 or 10 mg did not increase QTc versus placebo. In a one week clinical trial (N=86) of Cetirizine Hydrochloride Oral Solution, USP (0.25 mg/kg bid) compared with placebo in pediatric patients 6 to 11 months of age, ECG measurements taken within 3 hours of the last dose did not show any ECG abnormalities or increases in QTc interval in either group compared to baseline assessments. Data from other studies where cetirizine hydrochloride was administered to patients 6 to 23 months of age were consistent with the findings in this study.
The effects of cetirizine hydrochloride on the QTc interval at doses higher than 10 mg have not been studied in children less than 12 years of age.
In a six-week, placebo-controlled study of 186 patients (aged 12 to 64 years) with allergic rhinitis and mild to moderate asthma, cetirizine hydrochloride 10 mg once daily improved rhinitis symptoms and did not alter pulmonary function. In a two-week, placebo-controlled clinical trial, a subset analysis of 65 pediatric (aged 6 to 11 years) allergic rhinitis patients with asthma showed cetirizine hydrochloride did not alter pulmonary function. These studies support the safety of administering cetirizine hydrochloride to pediatric and adult allergic rhinitis patients with mild to moderate asthma.