Cevimeline
Cevimeline Prescribing Information
Cevimeline hydrochloride capsules are indicated for the treatment of symptoms of dry mouth in patients with Sjögren’s Syndrome.
The recommended dose of cevimeline hydrochloride capsules is 30 mg taken three times a day. There is insufficient safety information to support doses greater than 30 mg tid. There is also insufficient evidence for additional efficacy of cevimeline hydrochloride at doses greater than
30 mg tid.
Cevimeline is contraindicated in patients with uncontrolled asthma, known hypersensitivity to cevimeline, and when miosis is undesirable, e.g., in acute iritis and in narrow-angle (angle-closure) glaucoma.
Cevimeline was administered to 1777 patients during clinical trials worldwide, including Sjögren’s patients and patients with other conditions. In placebo-controlled Sjögren’s studies in the U.S., 320 patients received cevimeline doses ranging from 15 mg tid to 60 mg tid, of whom 93% were women and 7% were men. Demographic distribution was 90% Caucasian, 5% Hispanic, 3% Black and 2% of other origin. In these studies, 14.6% of patients discontinued treatment with cevimeline due to adverse events.
The following adverse events associated with muscarinic agonism were observed in the clinical trials of cevimeline in Sjögren’s syndrome patients:
Adverse Event | Cevimeline 30 mg (tid) n*=533 | Placebo (tid) n=164 |
| Excessive Sweating | 18.7% | 2.4% |
| Nausea | 13.8% | 7.9% |
| Rhinitis | 11.2% | 5.4% |
| Diarrhea | 10.3% | 10.3% |
| Excessive Salivation | 2.2% | 0.6% |
| Urinary Frequency | 0.9% | 1.8% |
| Asthenia | 0.5% | 0.0% |
| Flushing | 0.3% | 0.6% |
| Polyuria | 0.1% | 0.6% |
* n Is the total number of patients exposed to the dose at any time during the study.
In addition, the following adverse events (≥3% incidence) were reported in the Sjögren’s clinical trials:
Adverse Event | Cevimeline 30 mg (tid) n*=533 | Placebo (tid) n=164 |
| Headache | 14.4% | 20.1% |
| Sinusitis | 12.3% | 10.9% |
| Upper Respiratory Tract Infection | 11.4% | 9.1% |
| Dyspepsia | 7.8% | 8.5% |
| Abdominal Pain | 7.6% | 6.7% |
| Urinary Tract Infection | 6.1% | 3.0% |
| Coughing | 6.1% | 3.0% |
| Pharyngitis | 5.2% | 5.4% |
| Vomiting | 4.6% | 2.4% |
| Injury | 4.5% | 2.4% |
| Back Pain | 4.5% | 4.2% |
| Rash | 4.3% | 6.0% |
| Conjunctivitis | 4.3% | 3.6% |
| Dizziness | 4.1% | 7.3% |
| Bronchitis | 4.1% | 1.2% |
| Arthralgia | 3.7% | 1.8% |
| Surgical Intervention | 3.3% | 3.0% |
| Fatigue | 3.3% | 1.2% |
| Pain | 3.3% | 3.0% |
| Skeletal Pain | 2.8% | 1.8% |
| Insomnia | 2.4% | 1.2% |
| Hot Flushes | 2.4% | 0.0% |
| Rigors | 1.3% | 1.2% |
| Anxiety | 1.3% | 1.2% |
* n is the total number of patients exposed to the dose at any time during the study.
The following events were reported in Sjögren’s patients at incidences of <3% and ≥1%: constipation, tremor, abnormal vision, hypertonia, peripheral edema, chest pain, myalgia, fever, anorexia, eye pain, earache, dry mouth, vertigo, salivary gland pain, pruritus, influenza-like symptoms, eye infection, post operative pain, vaginitis, skin disorder, depression, hiccup, hyporeflexia, infection, fungal infection, sialoadenitis, otitis media, erythematous rash, pneumonia, edema, salivary gland enlargement, allergy, gastroesophageal reflux, eye abnormality, migraine, tooth disorder, epistaxis, flatulence, toothache, ulcerative stomatitis, anemia, hypoesthesia, cystitis, leg cramps, abscess, eructation, moniliasis, palpitation, increased amylase, xerophthalmia, allergic reaction.
The following events were reported rarely in treated Sjögren’s patients (<1%): Causal relation is unknown:
In one subject with lupus erythematosus receiving concomitant multiple drug therapy, a highly elevated ALT level was noted after the fourth week of cevimeline therapy. In two other subjects receiving cevimeline in the clinical trials, very high AST levels were noted. The significance of these findings is unknown.
Additional adverse events (relationship unknown) which occurred in other clinical studies (patient population different from Sjögren’s patients) are as follows:
cholinergic syndrome, blood pressure fluctuation, cardiomegaly, postural hypotension, aphasia, convulsions, abnormal gait, hyperesthesia, paralysis, abnormal sexual function, enlarged abdomen, change in bowel habits, gum hyperplasia, intestinal obstruction, bundle branch block, increased creatine phosphokinase, electrolyte abnormality, glycosuria, gout, hyperkalemia, hyperproteinemia, increased lactic dehydrogenase (LDH), increased alkaline phosphatase, failure to thrive, abnormal platelets, aggressive reaction, amnesia, apathy, delirium, delusion, dementia, illusion, impotence, neurosis, paranoid reaction, personality disorder, hyperhemoglobinemia, apnea, atelectasis, yawning, oliguria, urinary retention, distended vein, lymphocytosis.
Cevimeline is cis -2’-methylspiro{1-azabicyclo [2.2.2] octane-3, 5’-[1,3] oxathiolane} hydrochloride, hydrate (2:1). Its empirical formula is C10H17NOS.HCl.½ H2O, and its structural formula is:
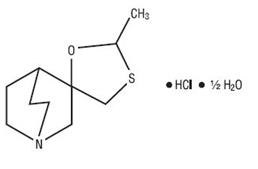
Cevimeline has a molecular weight of 244.79. It is a white to off white crystalline powder with a melting point range of 201 to 203oC. It is freely soluble in alcohol and chloroform, very soluble in water, and virtually insoluble in ether. The pH of a 1% solution ranges from 4.6 to 5.6. Inactive ingredients include lactose monohydrate, hydroxypropyl cellulose, and magnesium stearate.
Cevimeline is a cholinergic agonist which binds to muscarinic receptors. Muscarinic agonists in sufficient dosage can increase secretion of exocrine glands, such as salivary and sweat glands and increase tone of the smooth muscle in the gastrointestinal and urinary tracts.
Absorption: After administration of a single 30 mg capsule, cevimeline was rapidly absorbed with a mean time to peak concentration of 1.5 to 2 hours. No accumulation of active drug or its metabolites was observed following multiple dose administration. When administered with food, there is a decrease in the rate of absorption, with a fasting tMAX of 1.53 hours and a tMAX of 2.86 hours after a meal; the peak concentration is reduced by 17.3%. Single oral doses across the clinical dose range are dose proportional.
Distribution: Cevimeline has a volume of distribution of approximately 6L/kg and is <20% bound to human plasma proteins. This suggests that cevimeline is extensively bound to tissues; however, the specific binding sites are unknown.
Metabolism: Isozymes CYP2D6 and CYP3A3/4 are responsible for the metabolism of cevimeline. After 24 hours, 86.7% of the dose was recovered (16.0% unchanged, 44.5% as cis and trans-sulfoxide, 22.3% of the dose as glucuronic acid conjugate and 4% of the dose as N-oxide of cevimeline). Approximately 8% of the trans-sulfoxide metabolite is then
Excretion: The mean half-life of cevimeline is
Special Populations: The effects of renal impairment, hepatic impairment, or ethnicity on the pharmacokinetics of cevimeline have not been investigated.
Cevimeline has been shown to improve the symptoms of dry mouth in patients with Sjögren’s Syndrome.
A 6-week, randomized, double blind, placebo-controlled study was conducted in 75 patients
(10 men, 65 women) with a mean age of 53.6 years (range 33-75). The racial distribution was Caucasian 92%, Black 1 % and other 7%. The effects of cevimeline at 30 mg tid (90 mg/day) and 60 mg tid (180 mg/day) were compared to those of placebo. Patients were evaluated by a measure called global improvement, which is defined as a response of "better" to the question, "Please rate the overall condition of your dry mouth now compared with how you felt before starting treatment in this study." Patients also had the option of selecting "worse" or "no change" as answers. Seventy-six percent of the patients in the 30 mg tid group reported a global improvement in their dry mouth symptoms compared to 35% of the patients in the placebo group. This difference was statistically significant at p=0.0043. There was no evidence that patients in the 60 mg tid group had better global evaluation scores than the patients in the 30 mg tid group.
A 12-week, randomized, double-blind, placebo-controlled study was conducted in 197 patients (10 men, 187 women) with a mean age of 54.5 years (range 23-74). The racial distribution was Caucasian 91.4%, Black 3% and other 5.6%. The effects of cevimeline at 15 mg tid (45 mg/day) and 30 mg tid (90 mg/day) were compared to those of placebo. Statistically significant global improvement in the symptoms of dry mouth (p=0.0004) was seen for the 30 mg tid group compared to placebo, but not for the 15 mg group compared to placebo. Salivary flow showed statistically significant increases at both doses of cevimeline during the study compared to placebo.
A second 12-week, randomized, double-blind, placebo-controlled study was conducted in 212 patients (11 men, 201 women) with a mean age of 55.3 years (range 24-75). The racial distribution was Caucasian 88.7%, Black 1.9% and other 9.4%. The effects of cevimeline at 15 mg tid (45 mg/day) and 30 mg tid (90 mg/day) were compared to those of placebo. No statistically significant differences were noted in the patient global evaluations. However, there was a higher placebo response rate in this study compared to the aforementioned studies. The 30 mg tid group showed a statistically significant increase in salivary flow from pre-dose to
post-dose compared to placebo (p=0.0017).