Dexamethasone
Dexamethasone Prescribing Information
Control of severe or incapacitating allergic conditions intractable to adequate trials of conventional treatment in asthma, atopic dermatitis, contact dermatitis, drug hypersensitivity reactions, perennial or seasonal allergic rhinitis, and serum sickness.
Bullous dermatitis herpetiformis, exfoliative erythroderma, mycosis fungoides, pemphigus, and severe erythema multiforme (Stevens-Johnson syndrome).
Primary or secondary adrenocortical insufficiency (hydrocortisone or cortisone is the drug of choice; may be used in conjunction with synthetic mineralocorticoid analogs where applicable; in infancy mineralocorticoid supplementation is of particular importance), congenital adrenal hyperplasia, hypercalcemia associated with cancer, and nonsuppurative thyroiditis.
To tide the patient over a critical period of the disease in regional enteritis and ulcerative colitis.
Acquired (autoimmune) hemolytic anemia, congenital (erythroid) hypoplastic anemia (Diamond-Blackfan anemia), idiopathic thrombocytopenic purpura in adults, pure red cell aplasia, and selected cases of secondary thrombocytopenia.
Diagnostic testing of adrenocortical hyperfunction, trichinosis with neurologic or myocardial involvement, tuberculous meningitis with subarachnoid block or impending block when used with appropriate antituberculous chemotherapy.
For the palliative management of leukemias and lymphomas.
Acute exacerbations of multiple sclerosis, cerebral edema associated with primary or metastatic brain tumor, craniotomy, or head injury.
Sympathetic ophthalmia, temporal arteritis, uveitis, and ocular inflammatory conditions unresponsive to topical corticosteroids.
To induce a diuresis or remission of proteinuria in idiopathic nephrotic syndrome or that due to lupus erythematosus.
Berylliosis, fulminating or disseminated pulmonary tuberculosis when used concurrently with appropriate antituberculous chemotherapy, idiopathic eosinophilic pneumonias, symptomatic sarcoidosis.
As adjunctive therapy for short-term administration (to tide the patient over an acute episode or exacerbation) in acute gouty arthritis, acute rheumatic carditis, ankylosing spondylitis, psoriatic arthritis, rheumatoid arthritis, including juvenile rheumatoid arthritis (selected cases may require low-dose maintenance therapy). For the treatment of dermatomyositis, polymyositis, and systemic lupus erythematosus.
The initial dosage varies from 0.75 mg to 9 mg a day depending on the disease being treated.
After a favorable response is noted, the proper maintenance dosage should be determined by decreasing the initial drug dosage in small decrements at appropriate time intervals until the lowest dosage that maintains an adequate clinical response is reached.
Situations which may make dosage adjustments necessary are changes in clinical status secondary to remissions or exacerbations in the disease process, the patient’s individual drug responsiveness, and the effect of patient exposure to stressful situations not directly related to the disease entity under treatment. In this latter situation it may be necessary to increase the dosage of the corticosteroid for a period of time consistent with the patient’s condition. If after long-term therapy the drug is to be stopped, it is recommended that it be withdrawn gradually rather than abruptly.
In the treatment of acute exacerbations of multiple sclerosis, daily doses of 30 mg of dexamethasone for a week followed by 4 mg to 12 mg every other day for one month have been shown to be effective (see
Although controlled clinical trials have shown corticosteroids to be effective in speeding the resolution of acute exacerbations of multiple sclerosis, they do not show that they affect the ultimate outcome or natural history of the disease. The studies do show that relatively high doses of corticosteroids are necessary to demonstrate a significant effect (see
An acute myopathy has been observed with the use of high doses of corticosteroids, most often occurring in patients with disorders of neuromuscular transmission (e.g., myasthenia gravis), or in patients receiving concomitant therapy with neuromuscular blocking drugs (e.g., pancuronium). This acute myopathy is generalized, may involve ocular and respiratory muscles, and may result in quadriparesis. Elevation of creatinine kinase may occur. Clinical improvement or recovery after stopping corticosteroids may require weeks to years.
Psychic derangements may appear when corticosteroids are used, ranging from euphoria, insomnia, mood swings, personality changes, and severe depression, to frank psychotic manifestations. Also, existing emotional instability or psychotic tendencies may be aggravated by corticosteroids.
Although controlled clinical trials have shown corticosteroids to be effective in speeding the resolution of acute exacerbations of multiple sclerosis, they do not show that they affect the ultimate outcome or natural history of the disease. The studies do show that relatively high doses of corticosteroids are necessary to demonstrate a significant effect (see
An acute myopathy has been observed with the use of high doses of corticosteroids, most often occurring in patients with disorders of neuromuscular transmission (e.g., myasthenia gravis), or in patients receiving concomitant therapy with neuromuscular blocking drugs (e.g., pancuronium). This acute myopathy is generalized, may involve ocular and respiratory muscles, and may result in quadriparesis. Elevation of creatinine kinase may occur. Clinical improvement or recovery after stopping corticosteroids may require weeks to years.
Psychic derangements may appear when corticosteroids are used, ranging from euphoria, insomnia, mood swings, personality changes, and severe depression, to frank psychotic manifestations. Also, existing emotional instability or psychotic tendencies may be aggravated by corticosteroids.
In pediatric patients, the initial dose of dexamethasone may vary depending on the specific disease entity being treated. The range of initial doses is 0.02 mg to 0.3 mg/kg/day in three or four divided doses (0.6 mg to 9 mg/m2bsa/day).
Cortisone, 25 mg | Triamcinolone, 4 mg |
Hydrocortisone, 20 mg | Paramethasone, 2 mg |
Prednisolone, 5 mg | Betamethasone, 0.75 mg |
Prednisone, 5 mg | Dexamethasone, 0.75 mg |
Methylprednisolone, 4 mg |
1 mL or 2 mL, intramuscularly
4 tablets in two divided doses
4 tablets in two divided doses
2 tablets in two divided doses
1 tablet
1 tablet
No treatment
Follow-up visit
This schedule is designed to ensure adequate therapy during acute episodes, while minimizing the risk of overdosage in chronic cases.
In
1. Tests for Cushing's syndrome
Give 1 mg of dexamethasone orally at 11:00 p.m. Blood is drawn for plasma cortisol determination at 8:00 a.m. the following morning.
For greater accuracy, give 0.5 mg of dexamethasone orally every 6 hours for 48 hours. Twenty-four hour urine collections are made for determination of 17-hydroxycorticosteroid excretion.
2. Test to distinguish Cushing's syndrome due to pituitary ACTH excess from Cushing's syndrome due to other causes.
Give 2 mg of dexamethasone orally every 6 hours for 48 hours. Twenty-four hour urine collections are made for determination of 17-hydroxycorticosteroid excretion.
Systemic fungal infections (see
Dexamethasone tablets are contraindicated in patients who are hypersensitive to any components of this product.
The following adverse reactions have been reported with dexamethasone or other corticosteroids:
Anaphylactoid reaction, anaphylaxis, angioedema.
Bradycardia, cardiac arrest, cardiac arrhythmias, cardiac enlargement, circulatory collapse, congestive heart failure, fat embolism, hypertension, hypertrophic cardiomyopathy in premature infants, myocardial rupture following recent myocardial infarction (see
Average and large doses of corticosteroids can cause elevation of blood pressure, sodium and water retention, and increased excretion of potassium. These effects are less likely to occur with the synthetic derivatives except when used in large doses. Dietary salt restriction and potassium supplementation may be necessary. All corticosteroids increase calcium excretion.
Literature reports suggest an apparent association between use of corticosteroids and left ventricular free wall rupture after a recent myocardial infarction; therefore, therapy with corticosteroids should be used with great caution in these patients.
Average and large doses of corticosteroids can cause elevation of blood pressure, sodium and water retention, and increased excretion of potassium. These effects are less likely to occur with the synthetic derivatives except when used in large doses. Dietary salt restriction and potassium supplementation may be necessary. All corticosteroids increase calcium excretion.
Literature reports suggest an apparent association between use of corticosteroids and left ventricular free wall rupture after a recent myocardial infarction; therefore, therapy with corticosteroids should be used with great caution in these patients.
Acne, allergic dermatitis, dry scaly skin, ecchymoses and petechiae, erythema, impaired wound healing, increased sweating, rash, striae, suppression of reactions to skin tests, thin fragile skin, thinning scalp hair, urticaria.
Decreased carbohydrate and glucose tolerance, development of cushingoid state, hyperglycemia, glycosuria, hirsutism, hypertrichosis, increased requirements for insulin or oral hypoglycemic agents in diabetes, manifestations of latent diabetes mellitus, menstrual irregularities, secondary adrenocortical and pituitary unresponsiveness (particularly in times of stress, as in trauma, surgery, or illness), suppression of growth in pediatric patients.
Congestive heart failure in susceptible patients, fluid retention, hypokalemic alkalosis, potassium loss, sodium retention, tumor lysis syndrome.
Abdominal distention, elevation in serum liver enzyme levels (usually reversible upon discontinuation), hepatomegaly, increased appetite, nausea, pancreatitis, peptic ulcer with possible perforation and hemorrhage, perforation of the small and large intestine (particularly in patients with inflammatory bowel disease), ulcerative esophagitis.
Negative nitrogen balance due to protein catabolism.
Aseptic necrosis of femoral and humeral heads, loss of muscle mass, muscle weakness, osteoporosis, pathologic fracture of long bones, steroid myopathy, tendon rupture, vertebral compression fractures.
Convulsions, depression, emotional instability, euphoria, headache, increased intracranial pressure with papilledema (pseudotumor cerebri) usually following discontinuation of treatment, insomnia, mood swings, neuritis, neuropathy, paresthesia, personality changes, psychic disorders, vertigo.
Exophthalmos, glaucoma, increased intraocular pressure, posterior subcapsular cataracts, vision blurred.
Abnormal fat deposits, decreased resistance to infection, hiccups, increased or decreased motility and number of spermatozoa, malaise, moon face, weight gain.
Dexamethasone Tablets, USP are available for oral administration containing 2 mg of dexamethasone, USP. Each tablet contains the following inactive ingredients: corn starch, lactose monohydrate, magnesium stearate, pregelatinized starch and sodium starch glycolate type A.
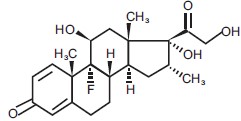
Dexamethasone, USP, a synthetic adrenocortical steroid, is a white or practically white crystalline powder. It is stable in air. It is sparingly soluble in acetone, ethanol, dioxane and methanol, slightly soluble in chloroform, practically insoluble in water.
The molecular formula is C22H29FO5. The molecular weight is 392.5 g/mol. It is designated chemically as (11β,16α)-9-fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione and the structural formula is:
Meets USP Dissolution Test 2.