Diphenhydramine
(Diphenhydramine Hydrochloride)Diphenhydramine Prescribing Information
Diphenhydramine hydrochloride in the injectable form is effective in adults and pediatric patients, other than premature infants and neonates, for the following conditions when diphenhydramine hydrochloride in the oral form is impractical.
THIS PRODUCT IS FOR INTRAVENOUS OR INTRAMUSCULAR ADMINISTRATION ONLY.
Diphenhydramine hydrochloride in the injectable form is indicated when the oral form is impractical.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
DOSAGE SHOULD BE INDIVIDUALIZED ACCORDING TO THE NEEDS AND THE RESPONSE OF THE PATIENT.
This drug should
1.
2.
3.
4.
5.
6.
7.
Diphenhydramine hydrochloride has additive effects with alcohol and other CNS depressants (hypnotics, sedatives, tranquilizers, etc).
MAO inhibitors prolong and intensify the anticholinergic (drying) effects of antihistamines.
Diphenhydramine hydrochloride USP is an antihistamine drug having the chemical name 2-(Diphenylmethoxy)-N,N-dimethylethylamine hydrochloride. It occurs as a white, crystalline powder, is freely soluble in water and alcohol and has a molecular weight of 291.82. The molecular formula is C
17H
21NO • HCI. The structural formula is as follows: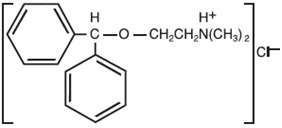
Diphenhydramine hydrochloride USP in the parenteral form is a sterile, pyrogen-free solution available in a concentration of 50 mg of diphenhydramine hydrochloride USP per mL. The solutions for parenteral use have been adjusted to a pH between 4 and 6.5 with either sodium hydroxide or hydrochloric acid.