Eloctate
(efmoroctocog alfa)Dosage & Administration
For intravenous use after reconstitution only.
By using PrescriberAI, you agree to the AI Terms of Use.
Eloctate Prescribing Information
ELOCTATE, Antihemophilic Factor (Recombinant), Fc Fusion Protein, is a recombinant DNA derived, antihemophilic factor indicated in adults and children with Hemophilia A (congenital Factor VIII deficiency) for:
- On-demand treatment and control of bleeding episodes,
- Perioperative management of bleeding,
- Routine prophylaxis to reduce the frequency of bleeding episodes.
Limitation of Use
ELOCTATE is not indicated for the treatment of von Willebrand disease.
For intravenous use after reconstitution only.
Dose
- Dose and duration of treatment depend on the severity of the Factor VIII deficiency, the location and extent of bleeding, and the patient's clinical condition. Careful monitoring of replacement therapy is necessary in cases of major surgery or life-threatening bleeding episodes.
- Each vial label of ELOCTATE states the Factor VIII potency in international units (IU). One IU corresponds to the activity of Factor VIII contained in one milliliter of normal human plasma.
- Potency assignment is determined using a chromogenic substrate assay. A field study1 has indicated that plasma Factor VIII levels can be monitored using either a chromogenic substrate assay or a one stage clotting assay routinely used in US clinical laboratories.
- Calculation of the required dose of Factor VIII is based on the empirical finding that 1 IU of Factor VIII per kg body weight raises the plasma Factor VIII level by 2 IU/dL.
The expected in vivo peak increase in Factor VIII level expressed as IU/dL (or % of normal) is estimated using the following formula:
Estimated Increment of Factor VIII (IU/dL or % of normal) = [Total Dose (IU)/body weight (kg)] × 2 (IU/dL per IU/kg)
The dose to achieve a desired in vivo peak increase in Factor VIII level may be calculated using the following formula:
Dose (IU) = body weight (kg) × Desired Factor VIII Rise (IU/dL or % of normal) × 0.5 (IU/kg per IU/dL)
- Patients may vary in their pharmacokinetic (e.g., half-life, in vivo recovery) and clinical responses. Base the dose and frequency of ELOCTATE on the individual clinical response.
- Dose adjustment may be necessary in pediatric patients under six years of age [see Use in Specific Populations (8.4)]. For patients six years of age or older, dose adjustment is not usually required.
On-demand Treatment and Control of Bleeding Episodes
A guide for dosing ELOCTATE for the on-demand treatment and control of bleeding episodes is provided in Table 1. Consideration should be given to maintaining a Factor VIII activity at or above the target range.
| Type of Bleeding | Factor VIII Level Required (IU/dL or % of normal) | Dose (IU/kg) | Frequency of Dosing (hours) | Duration of Therapy (days) |
|---|---|---|---|---|
| Minor and Moderate Joint, superficial muscle/no neurovascular compromise (except iliopsoas), deep laceration and renal, superficial soft tissue, mucous membranes | 40-60 | 20-30 | Repeat every 24-48 hours (12 to 24 hours for patients less than 6 years of age) | Until the bleeding episode is resolved |
| Major Life or limb threatening hemorrhage, iliopsoas and deep muscle with neurovascular injury, retroperitoneum, intracranial, or gastrointestinal | 80-100 | 40-50 | Repeat every 12-24 hours (8 to 24 hours for patients less than 6 years of age) | Until bleeding is resolved (approximately 7-10 days) |
Perioperative Management
A guide for dosing ELOCTATE during surgery (perioperative management) is provided in Table 2. Consideration should be given to maintaining a Factor VIII activity at or above the target range.
| Type of Surgery | Factor VIII Level Required (IU/dL or % of normal) | Dose (IU/kg) | Frequency of Dosing (hours) | Duration of Therapy (days) |
|---|---|---|---|---|
| Minor Uncomplicated tooth extraction | 50-80 | 25-40 | Repeat every 24 hours (12-24 hours for patients less than 6 years of age) | At least 1 day until healing is achieved |
| Major Intracranial, intra-abdominal, or joint replacement surgery | 80-120 (pre and postoperative) | Preoperative: 40-60 Repeat: 40-50 | Preoperative dose of 40 to 60 IU/kg followed by a repeat dose of 40-50 IU/kg after 8-24 hours (6 to 24 for patients less than 6 years of age) and then every 24 hours to maintain FVIII activity within the target range | Until adequate wound healing, then continue therapy for at least 7 days to maintain a Factor VIII activity within the target range |
Routine Prophylaxis
- The recommended starting regimen is 50 IU/kg of ELOCTATE administered every 4 days. Adjust the regimen based on patient response with dosing in the range of 25-65 IU/kg at 3-5 day intervals.
- For children <6 years of age, the recommended starting regimen is 50 IU/kg of ELOCTATE administered twice weekly. Adjust the regimen based on patient response with dosing in the range of 25-65 IU/kg at 3-5 day intervals. More frequent or higher doses up to 80 IU/kg may be required [see Use In Specific Populations (8.4), Clinical Pharmacology (12.3)].
Preparation and Reconstitution
- Use aseptic technique (clean and germ free) and a flat work surface during the reconstitution procedure.
- Allow the vial of ELOCTATE, containing the white to off-white lyophilized powder, and the pre-filled diluent syringe to reach room temperature before use.
- Remove the plastic cap from the vial and wipe the rubber stopper of the vial with an alcohol wipe. Allow the rubber stopper to dry.
Completely remove the backing from the vial adapter package by peeling back the lid. Do not remove the vial adapter from the package or touch the inside of the package of the adapter.
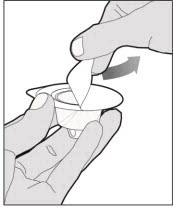
Place the vial on a flat and solid surface and use one hand to hold the vial steady. Use the other hand to place the vial adapter over the vial. Place the adapter spike directly above the center of the rubber stopper and push the adapter straight down until the spike punctures the center of the vial stopper and is fully inserted.
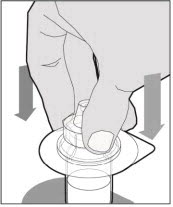
Lift the package cover away from the vial adapter and discard the cover.
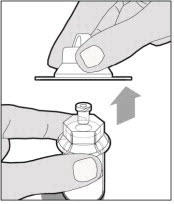
Hold the plunger rod at the circular disk. Place the tip of the plunger rod into the end of the syringe. Turn clockwise until it is securely attached. Only use the diluent syringe provided in the ELOCTATE package.
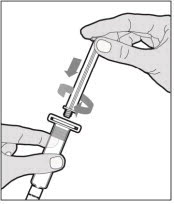
- With one hand, hold the diluent syringe by the ridged part directly under the cap, with the cap pointing up. Do not use if the cap has been removed or is not securely attached.
- With your other hand, grasp the cap and bend it at a 90° angle until it snaps off. After the cap snaps off, you will see the glass tip of the syringe. Do not touch the glass tip of the syringe or the inside of the cap.
- With the vial sitting on a flat surface, insert the tip of the syringe into the adapter opening. Turn the syringe clockwise until it is securely attached to the adapter.
- Slowly depress the plunger rod to inject all of the diluent into the vial. The plunger rod may rise slightly after this process. This is normal.
- With the syringe still connected to the adapter, gently swirl the vial until the product is completely dissolved. Do not shake. The reconstituted solution should be clear to slightly opalescent and colorless. Do not use the reconstituted ELOCTATE if it contains visible particles or is cloudy.
- Make sure the plunger rod is completely depressed. Turn the vial upside-down. Slowly pull on the plunger rod to draw the solution into the syringe. Be careful not to pull the plunger rod completely out of the syringe.
- Gently unscrew the syringe from the vial adapter and dispose of the vial with the adapter still attached. Do not touch the syringe tip or the inside of the cap.
- Use the reconstituted ELOCTATE as soon as possible, but no later than 3 hours after reconstitution. Do not touch the glass tip of the syringe if not used immediately after reconstitution. Protect from direct sunlight. Do not refrigerate after reconstitution.
To combine two or more vials of ELOCTATE, after step 12 above, follow these pooling steps:
- Remove the diluent syringe from the vial adapter by turning it counterclockwise until it is completely detached.
- Leave the vial adapter attached to the vial, as it is needed for attaching a large luer lock syringe (not included in kit). Do not detach the diluent syringe until ready to attach the large luer-lock syringe.
- Attach a separate, large luer-lock syringe by turning clockwise until it is securely in place.
- Slowly pull on the plunger rod to draw the solution into the syringe.
- Repeat this pooling procedure with each vial that is needed to obtain the required dose. When pooling, do not detach the large luer-lock syringe until ready to attach it to the next vial (with vial adapter attached). Once you have pooled the required dose, proceed to administration using the large luer-lock syringe.
Administration
For intravenous injection only
- Inspect the reconstituted ELOCTATE solution visually for particulate matter and discoloration prior to administration. Do not use if particulate matter or discoloration is observed.
- Do not administer reconstituted ELOCTATE in the same tubing or container with other medications.
Administration Steps:
- Attach the syringe to the connector end of the infusion set tubing by turning clockwise until it is securely in place.
- Depress the plunger until all air is removed from the syringe and ELOCTATE has reached the end of the infusion set tubing. Do not push ELOCTATE solution through the needle.
- Remove the protective needle cover from the infusion set tubing.
- Perform intravenous bolus infusion. The rate of administration should be determined by the patient's comfort level, and no faster than 10 ml per minute. After infusing ELOCTATE, remove and properly discard the infusion set.
ELOCTATE is available as a white to off-white lyophilized powder in single-dose vials containing nominally 250, 500, 750, 1000, 1500, 2000, 3000, 4000, 5000 or 6000 international units (IU) per vial. The actual Factor VIII potency is labeled on each ELOCTATE vial.
Pregnancy
Risk Summary
There are no studies of ELOCTATE use in pregnant women to inform a drug-associated risk. The background risk of major birth defects and miscarriage in the indicated population is unknown; however, the background risk of major birth defects in the U.S. general population is 2-4% and of miscarriage is 15-20% of clinically recognized pregnancies.
Animal reproductive and developmental toxicity studies have not been conducted with ELOCTATE. In a placental transfer study, ELOCTATE was detected in murine fetal blood samples at approximately 1% of the maternal blood levels (range, 0.2% to 1.9%), 3 to 4 hours following dosing of pregnant mice with 260 to 650 times the clinical dose of 20 to 50 IU/kg ELOCTATE [see Data].
It is not known whether ELOCTATE can cause fetal harm when administered to a pregnant woman, or whether it can affect reproduction capacity. If ELOCTATE is clearly needed to treat a pregnant woman, advise the patient that the risks to the mother and to the fetus are unknown.
Data
Animal data
Pregnant, genetically-modified, FVIII-deficient mice (Hem A mice) were injected intravenously with a single dose of 400 IU (approximately 13,000 IU/kg) ELOCTATE at the end of pregnancy on Gestation Day 19. Blood samples were collected from the maternal mice and the fetuses 3 to 4 hours after dosing, and FVIII activity was measured in both maternal and fetal plasma using a FVIII chromogenic assay. After dosing pregnant HemA mice with ELOCTATE, FVIII activity in fetal blood was approximately 1% of the maternal blood levels, suggesting that placental transfer of ELOCTATE may occur. The relevance of these data to humans is unknown.
Lactation
Risk Summary
There is no information regarding the presence of ELOCTATE in human milk, its effects on the breastfed infant, or its effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ELOCTATE and any potential adverse effects on the breastfed infant from ELOCTATE or from the underlying maternal condition.
Pediatric Use
Safety and efficacy studies have been performed in 82 previously treated, pediatric patients (PTPs) <18 years of age who received at least one dose of ELOCTATE as part of routine prophylaxis, on-demand treatment of bleeding episodes, or perioperative management. Adolescent subjects were enrolled in the adult and adolescent safety and efficacy trial, and subjects <12 were enrolled in a pediatric trial. Safety and efficacy of ELOCTATE have been evaluated in 103 previously untreated pediatric patients (PUPs) <6 years of age (median 0.58 year; range: 0.02–4 years) in one study (PUPs Study) [see Adverse Reactions (6)].
Pharmacokinetic data from a pediatric study of the 54 evaluable subjects <12 years of age showed that no dose adjustment was required for patients ≥6 years old. Children age 1 to 5 years had a shorter half-life and higher clearance (adjusted for body weight); therefore, a higher dose or more frequent dosing may be needed in this age group [see Clinical Pharmacology (12.3)].
Geriatric Use
Clinical studies of ELOCTATE did not include sufficient numbers of subjects aged 65 and over to determine whether or not they respond differently from younger subjects.
ELOCTATE is contraindicated in patients who have had life-threatening hypersensitivity reactions to ELOCTATE or its excipients (sucrose, sodium chloride, L-histidine, calcium chloride and polysorbate 20).
Hypersensitivity Reactions
Hypersensitivity reactions have been reported with ELOCTATE. Allergic-type hypersensitivity reactions, including anaphylaxis, have been reported with Factor VIII replacement products. Early signs of hypersensitivity reactions that can progress to anaphylaxis may include angioedema, chest tightness, dyspnea, wheezing, urticaria, and pruritus. Immediately discontinue administration and initiate appropriate treatment if hypersensitivity reactions occur.
Neutralizing Antibodies
Formation of neutralizing antibodies (inhibitors) to Factor VIII has been reported following administration of ELOCTATE. Monitor all patients for the development of Factor VIII inhibitors by appropriate clinical observations and laboratory tests. If the plasma Factor VIII level fails to increase as expected or if bleeding is not controlled after ELOCTATE administration, suspect the presence of an inhibitor (neutralizing antibody) [see Warnings and Precautions (5.5)].
Cardiovascular Risk Factors
Hemophilic patients with cardiovascular risk factors or diseases may be at the same risk to develop cardiovascular events as non-hemophilic patients when clotting has been normalized by treatment with Factor VIII.
Catheter-Related Complication
If a central venous access device (CVAD) is required, risk of CVAD-related complications including local infections, bacteremia, and catheter-site thrombosis should be considered [see Adverse Reactions (6)].
Monitoring Laboratory Tests
Monitor plasma Factor VIII activity by performing a validated test (e.g., one stage clotting assay), to confirm that adequate Factor VIII levels have been achieved and maintained [see Dosage and Administration (2)].
Monitor for the development of Factor VIII inhibitors. Perform a Bethesda inhibitor assay if expected Factor VIII plasma levels are not attained, or if bleeding is not controlled with the expected dose of ELOCTATE. Use Bethesda Units (BU) to report inhibitor levels.