Get your patient on Entyvio (Vedolizumab)
Entyvio prior authorization resources
Most recent state uniform prior authorization forms
Brand Resources
Entyvio patient education
Patient toolkit
Patient Support Program
Dosage & administration
DOSAGE AND ADMINISTRATION
Important Administration Information
- Before initiating ENTYVIO, update immunizations according to current immunization guidelines. (2.1 , 5.5 )
- Intravenous Administration : ENTYVIO should be administered intravenously by a healthcare provider. (2.1 )
- Subcutaneous Injection : ENTYVIO prefilled syringe and ENTYVIO PEN are intended for subcutaneous use. A patient may self-inject or caregiver may inject after proper training on correct subcutaneous injection technique. (2.1 )
Recommended Dosage (2.2 )
- Week 0 : 300 mg infused intravenously over approximately 30 minutes.
- Week 2 : 300 mg infused intravenously over approximately 30 minutes.
- Week 6 : Patients may remain on ENTYVIO intravenous therapy or switch to subcutaneous injection after receiving two ENTYVIO intravenous doses administered at Week 0 and Week 2.
- Intravenous Infusion : 300 mg infused over approximately 30 minutes and then every eight weeks thereafter.
- Subcutaneous Injection : 108 mg subcutaneously once every two weeks.
- Discontinue ENTYVIO in patients who do not show evidence of therapeutic benefit by Week 14.
- Patients currently receiving and responding to ENTYVIO intravenous therapy after Week 6 may also be switched to subcutaneous injection. Administer the first subcutaneous dose in place of the next scheduled intravenous infusion and every two weeks thereafter.
Preparation and Administration Instructions:
Important Administration Information
Before initiating ENTYVIO, update immunizations according to current immunization guidelines [see Warnings and Precautions (5.5) ].
Intravenous Administration
- ENTYVIO should be administered by a healthcare provider prepared to manage hypersensitivity reactions including anaphylaxis, if they occur [see Warnings and Precautions (5.1) ] . Appropriate monitoring and medical support measures should be available for immediate use. Observe patients during infusion and until the infusion is complete.
- Reconstitute and dilute ENTYVIO lyophilized powder prior to administration as a 30-minute intravenous infusion [see Dosage and Administration (2.3) ].
Subcutaneous Injection
- ENTYVIO prefilled syringe and ENTYVIO PEN are intended for subcutaneous use under the guidance and supervision of a healthcare professional.
- Patients may self-inject or caregivers may inject subcutaneous ENTYVIO using either the ENTYVIO prefilled syringe or ENTYVIO PEN after training in subcutaneous injection technique. Provide proper training to patients and/or caregivers on the subcutaneous injection technique of ENTYVIO.
Recommended Dosage in Adults with Ulcerative Colitis and Crohn’s Disease
- Week 0: Administer ENTYVIO 300 mg by intravenous infusion over approximately 30 minutes [ see Dosage and Administration (2.3) ].
- Week 2: Administer ENTYVIO 300 mg by intravenous infusion over approximately 30 minutes.
- Week 6: Patients may remain on ENTYVIO intravenous therapy or switch to subcutaneous injection after receiving two ENTYVIO intravenous doses administered at Week 0 and Week 2.
- Intravenous Infusion : Administer ENTYVIO 300 mg by intravenous infusion over approximately 30 minutes and then every eight weeks thereafter.
- Subcutaneous Injection : Administer ENTYVIO 108 mg subcutaneously once every 2 weeks.
- Discontinue therapy in patients who show no evidence of therapeutic benefit by Week 14.
Patients currently receiving and responding to ENTYVIO intravenous therapy after Week 6 may also be switched to subcutaneous injection. Administer the first subcutaneous dose in place of the next scheduled intravenous infusion and every two weeks thereafter.
Preparation and Administration Instructions for Intravenous Infusion
Reconstitution Instructions
- Remove the flip-off cap from the single-dose vial and wipe with alcohol swab. Reconstitute ENTYVIO vial containing lyophilized powder with 4.8 mL of Sterile Water for injection, 0.9% Sodium Chloride Injection, or Lactated Ringer's Injection, at room temperature (20°C to 25°C [68ºF to 77ºF]), using a syringe with a 21- to 25-gauge needle.
- Insert the syringe needle into the vial through the center of the stopper and direct the stream of Sterile Water for Injection, 0.9% Sodium Chloride Injection, or Lactated Ringer's Injection, to the glass wall of the vial to avoid excessive foaming.
- Gently swirl the vial for at least 15 seconds to dissolve the lyophilized powder. Do not vigorously shake or invert.
- Allow the solution to sit for up to 20 minutes at room temperature to allow for reconstitution and for any foam to settle; the vial can be swirled and inspected for dissolution during this time. If not fully dissolved after 20 minutes, allow another 10 minutes for dissolution. Do not use the vial if the drug product is not dissolved within 30 minutes.
- Visually inspect the reconstituted ENTYVIO solution for particulate matter and discoloration prior to dilution. Solution should be clear or opalescent, colorless to light brownish yellow and free of visible particulates. Do not administer reconstituted solution showing uncharacteristic color or containing particulates.
- Once dissolved, gently invert vial three times.
- Immediately, withdraw 5 mL (300 mg) of reconstituted ENTYVIO solution using a syringe with a 21- to 25-gauge needle. Discard any remaining portion of the reconstituted solution in the vial.
Dilution Instructions
Add the 5 mL (300 mg) of reconstituted ENTYVIO solution to 250 mL of 0.9% Sodium Chloride Injection, or Lactated Ringer's Injection, and gently mix the infusion bag. Do not add other medicinal products to the prepared infusion solution or intravenous infusion set. Once reconstituted and diluted, use the infusion solution as soon as possible.
Discard any unused portion of the infusion solution .
Administration Instructions
After the infusion is complete, flush with 30 mL of 0.9% Sodium Chloride Injection, or Lactated Ringer's Injection.
Storage and Stability
Specific storage conditions and timing for the reconstituted solution in vial and diluted solution in the infusion bag are outlined in Table 1 .
Do not freeze the reconstituted solution in the vial or the diluted solution in the infusion bag.
| Solution | Storage Conditions | |
|---|---|---|
| Refrigeration (2°C to 8°C [36°F to 46°F]) | Room Temperature (20°C to 25°C [68°F to 77°F]) | |
| Reconstituted Solution (in Sterile Water for Injection, 0.9% Sodium Chloride Injection, or Lactated Ringer's Injection, inside vial) | 8 hours | Use immediately after reconstitution |
| Diluted Solution (in 0.9% Sodium Chloride Injection) | 24 hours This time assumes the reconstituted solution is immediately diluted in the 0.9% Sodium Chloride Injection, or Lactated Ringer's Injection, and held in the infusion bag only. Any time that the reconstituted solution was held in vial should be subtracted from the time the solution may be held in the infusion bag. , This period may include up to 12 hours at room temperature (20°C to 25°C [68°F to 77°F]). | 12 hours |
| Diluted Solution (in Lactated Ringer's Injection) | 6 hours | Use immediately after dilution |
The combined storage time of reconstituted ENTYVIO solution in the vial and the diluted solution in the infusion bag with 0.9% Sodium Chloride Injection, is a total of 12 hours at room temperature (20°C to 25°C [68°F to 77°F]) or 24 hours refrigerated (2°C to 8°C [36°F to 46°F]). This combined storage time may include up to eight hours of the reconstituted solution in the vial at 2°C to 8°C.
The combined storage time of reconstituted ENTYVIO solution in the vial and the diluted solution in the infusion bag with Lactated Ringer's Injection, is a total of six hours refrigerated (2°C to 8°C [36°F to 46°F]).
2.4 Preparation and Administration Instructions for Subcutaneous Injection
- Inspect the solution visually for particulate matter and discoloration prior to administration. ENTYVIO in prefilled syringe or ENTYVIO PEN should be a clear to moderately opalescent, colorless to slightly yellow solution. Do not use ENTYVIO prefilled syringes or ENTYVIO PENs with visible particulate matter or discoloration.
- Administer each subcutaneous injection at a different anatomic location (such as thighs, any quadrant of abdomen, or upper arms) than the previous injection. Administration of ENTYVIO in the back of upper arm may only be performed by a healthcare professional or caregiver. Do not inject into moles, scars, bruises, or areas where the skin is tender, erythematous, or indurated.
Missed Subcutaneous Dose
If treatment with subcutaneous ENTYVIO is interrupted or if a scheduled dose(s) of subcutaneous ENTYVIO is missed, inject the next subcutaneous dose as soon as possible and then every 2 weeks thereafter.
In the event of incomplete dose administration (i.e., patient attempts administration of dose with ENTYVIO PEN, however it is uncertain if a full dose was administered), instruct the patient to call their pharmacy or healthcare provider.

By using PrescriberAI, you agree to the AI Terms of Use.
Entyvio prescribing information
INDICATIONS AND USAGE
ENTYVIO is indicated in adults for the treatment of:
- moderately to severely active ulcerative colitis (UC).
- moderately to severely active Crohn's disease (CD).
DOSAGE AND ADMINISTRATION
Important Administration Information
- Before initiating ENTYVIO, update immunizations according to current immunization guidelines. (2.1 , 5.5 )
- Intravenous Administration : ENTYVIO should be administered intravenously by a healthcare provider. (2.1 )
- Subcutaneous Injection : ENTYVIO prefilled syringe and ENTYVIO PEN are intended for subcutaneous use. A patient may self-inject or caregiver may inject after proper training on correct subcutaneous injection technique. (2.1 )
Recommended Dosage (2.2 )
- Week 0 : 300 mg infused intravenously over approximately 30 minutes.
- Week 2 : 300 mg infused intravenously over approximately 30 minutes.
- Week 6 : Patients may remain on ENTYVIO intravenous therapy or switch to subcutaneous injection after receiving two ENTYVIO intravenous doses administered at Week 0 and Week 2.
- Intravenous Infusion : 300 mg infused over approximately 30 minutes and then every eight weeks thereafter.
- Subcutaneous Injection : 108 mg subcutaneously once every two weeks.
- Discontinue ENTYVIO in patients who do not show evidence of therapeutic benefit by Week 14.
- Patients currently receiving and responding to ENTYVIO intravenous therapy after Week 6 may also be switched to subcutaneous injection. Administer the first subcutaneous dose in place of the next scheduled intravenous infusion and every two weeks thereafter.
Preparation and Administration Instructions:
Important Administration Information
Before initiating ENTYVIO, update immunizations according to current immunization guidelines [see Warnings and Precautions (5.5) ].
Intravenous Administration
- ENTYVIO should be administered by a healthcare provider prepared to manage hypersensitivity reactions including anaphylaxis, if they occur [see Warnings and Precautions (5.1) ] . Appropriate monitoring and medical support measures should be available for immediate use. Observe patients during infusion and until the infusion is complete.
- Reconstitute and dilute ENTYVIO lyophilized powder prior to administration as a 30-minute intravenous infusion [see Dosage and Administration (2.3) ].
Subcutaneous Injection
- ENTYVIO prefilled syringe and ENTYVIO PEN are intended for subcutaneous use under the guidance and supervision of a healthcare professional.
- Patients may self-inject or caregivers may inject subcutaneous ENTYVIO using either the ENTYVIO prefilled syringe or ENTYVIO PEN after training in subcutaneous injection technique. Provide proper training to patients and/or caregivers on the subcutaneous injection technique of ENTYVIO.
Recommended Dosage in Adults with Ulcerative Colitis and Crohn’s Disease
- Week 0: Administer ENTYVIO 300 mg by intravenous infusion over approximately 30 minutes [ see Dosage and Administration (2.3) ].
- Week 2: Administer ENTYVIO 300 mg by intravenous infusion over approximately 30 minutes.
- Week 6: Patients may remain on ENTYVIO intravenous therapy or switch to subcutaneous injection after receiving two ENTYVIO intravenous doses administered at Week 0 and Week 2.
- Intravenous Infusion : Administer ENTYVIO 300 mg by intravenous infusion over approximately 30 minutes and then every eight weeks thereafter.
- Subcutaneous Injection : Administer ENTYVIO 108 mg subcutaneously once every 2 weeks.
- Discontinue therapy in patients who show no evidence of therapeutic benefit by Week 14.
Patients currently receiving and responding to ENTYVIO intravenous therapy after Week 6 may also be switched to subcutaneous injection. Administer the first subcutaneous dose in place of the next scheduled intravenous infusion and every two weeks thereafter.
Preparation and Administration Instructions for Intravenous Infusion
Reconstitution Instructions
- Remove the flip-off cap from the single-dose vial and wipe with alcohol swab. Reconstitute ENTYVIO vial containing lyophilized powder with 4.8 mL of Sterile Water for injection, 0.9% Sodium Chloride Injection, or Lactated Ringer's Injection, at room temperature (20°C to 25°C [68ºF to 77ºF]), using a syringe with a 21- to 25-gauge needle.
- Insert the syringe needle into the vial through the center of the stopper and direct the stream of Sterile Water for Injection, 0.9% Sodium Chloride Injection, or Lactated Ringer's Injection, to the glass wall of the vial to avoid excessive foaming.
- Gently swirl the vial for at least 15 seconds to dissolve the lyophilized powder. Do not vigorously shake or invert.
- Allow the solution to sit for up to 20 minutes at room temperature to allow for reconstitution and for any foam to settle; the vial can be swirled and inspected for dissolution during this time. If not fully dissolved after 20 minutes, allow another 10 minutes for dissolution. Do not use the vial if the drug product is not dissolved within 30 minutes.
- Visually inspect the reconstituted ENTYVIO solution for particulate matter and discoloration prior to dilution. Solution should be clear or opalescent, colorless to light brownish yellow and free of visible particulates. Do not administer reconstituted solution showing uncharacteristic color or containing particulates.
- Once dissolved, gently invert vial three times.
- Immediately, withdraw 5 mL (300 mg) of reconstituted ENTYVIO solution using a syringe with a 21- to 25-gauge needle. Discard any remaining portion of the reconstituted solution in the vial.
Dilution Instructions
Add the 5 mL (300 mg) of reconstituted ENTYVIO solution to 250 mL of 0.9% Sodium Chloride Injection, or Lactated Ringer's Injection, and gently mix the infusion bag. Do not add other medicinal products to the prepared infusion solution or intravenous infusion set. Once reconstituted and diluted, use the infusion solution as soon as possible.
Discard any unused portion of the infusion solution .
Administration Instructions
After the infusion is complete, flush with 30 mL of 0.9% Sodium Chloride Injection, or Lactated Ringer's Injection.
Storage and Stability
Specific storage conditions and timing for the reconstituted solution in vial and diluted solution in the infusion bag are outlined in Table 1 .
Do not freeze the reconstituted solution in the vial or the diluted solution in the infusion bag.
| Solution | Storage Conditions | |
|---|---|---|
| Refrigeration (2°C to 8°C [36°F to 46°F]) | Room Temperature (20°C to 25°C [68°F to 77°F]) | |
| Reconstituted Solution (in Sterile Water for Injection, 0.9% Sodium Chloride Injection, or Lactated Ringer's Injection, inside vial) | 8 hours | Use immediately after reconstitution |
| Diluted Solution (in 0.9% Sodium Chloride Injection) | 24 hours This time assumes the reconstituted solution is immediately diluted in the 0.9% Sodium Chloride Injection, or Lactated Ringer's Injection, and held in the infusion bag only. Any time that the reconstituted solution was held in vial should be subtracted from the time the solution may be held in the infusion bag. , This period may include up to 12 hours at room temperature (20°C to 25°C [68°F to 77°F]). | 12 hours |
| Diluted Solution (in Lactated Ringer's Injection) | 6 hours | Use immediately after dilution |
The combined storage time of reconstituted ENTYVIO solution in the vial and the diluted solution in the infusion bag with 0.9% Sodium Chloride Injection, is a total of 12 hours at room temperature (20°C to 25°C [68°F to 77°F]) or 24 hours refrigerated (2°C to 8°C [36°F to 46°F]). This combined storage time may include up to eight hours of the reconstituted solution in the vial at 2°C to 8°C.
The combined storage time of reconstituted ENTYVIO solution in the vial and the diluted solution in the infusion bag with Lactated Ringer's Injection, is a total of six hours refrigerated (2°C to 8°C [36°F to 46°F]).
2.4 Preparation and Administration Instructions for Subcutaneous Injection
- Inspect the solution visually for particulate matter and discoloration prior to administration. ENTYVIO in prefilled syringe or ENTYVIO PEN should be a clear to moderately opalescent, colorless to slightly yellow solution. Do not use ENTYVIO prefilled syringes or ENTYVIO PENs with visible particulate matter or discoloration.
- Administer each subcutaneous injection at a different anatomic location (such as thighs, any quadrant of abdomen, or upper arms) than the previous injection. Administration of ENTYVIO in the back of upper arm may only be performed by a healthcare professional or caregiver. Do not inject into moles, scars, bruises, or areas where the skin is tender, erythematous, or indurated.
Missed Subcutaneous Dose
If treatment with subcutaneous ENTYVIO is interrupted or if a scheduled dose(s) of subcutaneous ENTYVIO is missed, inject the next subcutaneous dose as soon as possible and then every 2 weeks thereafter.
In the event of incomplete dose administration (i.e., patient attempts administration of dose with ENTYVIO PEN, however it is uncertain if a full dose was administered), instruct the patient to call their pharmacy or healthcare provider.
DOSAGE FORMS AND STRENGTHS
Intravenous infusion
- For injection: 300 mg vedolizumab in a single-dose vial. (3 )
Subcutaneous injection
Intravenous Infusion
- For injection: 300 mg of vedolizumab as a white to off-white lyophilized cake in a single-dose vial for reconstitution.
Subcutaneous Injection
- Injection: 108 mg/0.68 mL vedolizumab as a clear to moderately opalescent, colorless to slightly yellow solution in a single-dose prefilled syringe with needle safety device.
- Injection: 108 mg/0.68 mL vedolizumab as a clear to moderately opalescent, colorless to slightly yellow solution in a single-dose prefilled pen (ENTYVIO PEN).
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
Available data from the Organization of Teratology Information Specialists (OTIS)/MotherToBaby ENTYVIO Pregnancy Registry, published literature and pharmacovigilance in pregnant women have not reliably identified an ENTYVIO-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes (see Data ) . There are risks to the mother and the fetus associated with inflammatory bowel disease in pregnancy (see Clinical Considerations ) .
No fetal harm was observed in animal reproduction studies with intravenous administration of vedolizumab to rabbits and monkeys at dose levels 20 times the recommended human dosage (see Data ) .
The background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and miscarriage is 15 to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and Embryo/Fetal Risk
Published data suggest that the risk of adverse pregnancy outcomes in women with inflammatory bowel disease (IBD) is associated with increased disease activity. Adverse pregnancy outcomes include preterm delivery (before 37 weeks of gestation), low birth weight (less than 2,500 g) infants, and small for gestational age at birth.
Fetal/Neonatal Adverse Reactions
ENTYVIO administered during pregnancy could affect immune responses in the in utero exposed newborn and infant. The clinical significance of low levels of ENTYVIO in utero-exposed infants is unknown. The safety of administering live or live-attenuated vaccines in exposed infants is unknown.
Data
Human Data
The vedolizumab pregnancy exposure registry conducted by OTIS/MotherToBaby study in the United States and Canada collected prospective observational data between 2015 and 2022 to assess the risk of major birth defects in live-born infants of women with ulcerative colitis (UC) or Crohn’s disease (CD) treated with vedolizumab during pregnancy. The study compared pregnant patients with UC or CD exposed to vedolizumab with pregnant patients with UC or CD treated with other biological products. The registry included 99 women (58 with UC, 41 with CD) treated with vedolizumab during pregnancy, and 76 women (27 with UC, 49 with CD) exposed to other biological products during pregnancy.
The proportion of major birth defects among live-born infants in patients with UC or CD treated with vedolizumab and patients with UC or CD treated with other biological products was 7.4% (7/94) and 5.6% (4/71), respectively. Overall, there was no evidence of increased risk for major structural birth defects (adjusted RR 1.07, 95% CI: 0.33, 3.52).
The methodological limitations of the registry, including small sample size and the non-randomized design, resulted in a limited ability to estimate the risk of major birth defects and other maternal and infant outcomes. The conclusions from the pregnancy registry were consistent with the published literature and pharmacovigilance.
Animal Data
A reproduction study has been performed in pregnant rabbits at single intravenous doses up to 100 mg/kg administered on gestation Day 7 (about 20 times the recommended human dosage) and has revealed no evidence of impaired fertility or harm to the fetus due to vedolizumab. A pre- and post-natal development study in monkeys showed no evidence of any adverse effect on pre- and post-natal development at intravenous doses up to 100 mg/kg (about 20 times the recommended human dosage).
Lactation
Risk Summary
Data from a clinical lactation study show the presence of vedolizumab in human milk. The mean calculated daily infant dosage was 0.02 mg/kg/day orally ( see Data ) . Systemic exposure in a breastfed infant is expected to be low because monoclonal antibodies are largely degraded in the gastrointestinal tract. There are no data on the effects of vedolizumab on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ENTYVIO and any potential adverse effects on the breastfed infant from ENTYVIO or from the underlying maternal condition.
Data
A milk-only lactation study was conducted in 9 adult lactating women being treated for active ulcerative colitis or Crohn's disease with intravenous ENTYVIO every 8 weeks after reaching steady state and completing the induction phase (ENTYVIO administration at 0, 2, and 6 weeks). Mean concentrations of ENTYVIO in human milk ranged from 0.03 to 0.26 mcg/mL. The mean calculated daily infant oral dosage was 0.02 mg/kg/day calculated as a product of the average concentration over the 8-week dosing interval and the standardized milk consumption of 150 mL/kg/day.
Pediatric Use
Safety and effectiveness of ENTYVIO in pediatric patients have not been established.
Geriatric Use
Clinical trials of ENTYVIO did not include sufficient numbers of patients aged 65 and over (72 patients with Crohn's disease or ulcerative colitis aged 65 and over were treated with ENTYVIO during controlled Phase 3 trials) to determine whether they respond differently from younger adult patients. However, no overall differences in safety or effectiveness were observed between these patients and younger adult patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients.
CONTRAINDICATIONS
ENTYVIO is contraindicated in patients who have had a known serious or severe hypersensitivity reaction to ENTYVIO or any of its excipients (such as dyspnea, bronchospasm, urticaria, flushing, rash and increased heart rate) [see Warnings and Precautions (5.1) ] .
WARNINGS AND PRECAUTIONS
- Infusion-Related Reactions and Hypersensitivity Reactions : Discontinue ENTYVIO and initiate appropriate treatment if serious reactions occur. (5.1 )
- Infections : Treatment with ENTYVIO is not recommended in patients with active, severe infections until the infections are controlled. Consider withholding ENTYVIO in patients who develop a severe infection while on treatment with ENTYVIO. (5.2 )
- Progressive Multifocal Leukoencephalopathy (PML) : Although unlikely, a risk of PML cannot be ruled out. Monitor patients for any new or worsening neurological signs or symptoms. (5.3 )
Infusion-Related Reactions and Hypersensitivity Reactions
Infusion-related reactions and hypersensitivity reactions have been reported, including anaphylaxis, dyspnea, bronchospasm, urticaria, flushing, rash, and increased blood pressure and heart rate [see Adverse Reactions (6.1 , 6.2) ]. These reactions may occur with the first or subsequent infusions of ENTYVIO and may vary in their time of onset from during infusion or up to several hours post-infusion.
If anaphylaxis or other serious infusion-related or hypersensitivity reactions occur, discontinue administration of ENTYVIO immediately and initiate appropriate treatment.
Infections
Patients treated with ENTYVIO are at increased risk for developing infections [see Adverse Reactions (6.1) ]. The most commonly reported infections in clinical trials occurring at a rate greater on ENTYVIO than placebo involved the upper respiratory and nasal mucosa (e.g., nasopharyngitis, upper respiratory tract infection). Serious infections have also been reported in patients treated with ENTYVIO, including anal abscess, sepsis (some fatal), tuberculosis, salmonella sepsis, Listeria meningitis, giardiasis and cytomegaloviral colitis.
ENTYVIO is not recommended in patients with active, severe infections until the infections are controlled. Consider withholding treatment in patients who develop a severe infection while on treatment with ENTYVIO. Exercise caution when considering the use of ENTYVIO in patients with a history of recurring severe infections. Consider screening for tuberculosis (TB) according to the local practice. For progressive multifocal leukoencephalopathy (PML), [see Warnings and Precautions (5.3) ] .
Progressive Multifocal Leukoencephalopathy
PML, a rare and often fatal opportunistic infection of the central nervous system (CNS), has been reported with systemic immunosuppressants, including another integrin receptor antagonist. PML is caused by the John Cunningham (JC) virus and typically only occurs in patients who are immunocompromised. One case of PML in an ENTYVIO-treated patient with multiple contributory factors has been reported in the postmarketing setting (e.g., human immunodeficiency virus [HIV] infection with a CD4 count of 300 cells/mm 3 and prior and concomitant immunosuppression). Although unlikely, a risk of PML cannot be ruled out.
Monitor patients on ENTYVIO for any new onset, or worsening, of neurological signs and symptoms. Typical signs and symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes. The progression of deficits usually leads to death or severe disability over weeks or months. If PML is suspected, withhold dosing with ENTYVIO and refer to a neurologist; if confirmed, discontinue dosing permanently.
Liver Injury
There have been reports of elevations of transaminase and/or bilirubin in patients receiving ENTYVIO. In general, the combination of transaminase elevations and elevated bilirubin without evidence of obstruction is generally recognized as an important predictor of severe liver injury that may lead to death or the need for a liver transplant in some patients. ENTYVIO should be discontinued in patients with jaundice or other evidence of significant liver injury [see Adverse Reactions (6.1) ] .
Live and Oral Vaccines
Prior to initiating treatment with ENTYVIO, all patients should be brought up to date with all immunizations according to current immunization guidelines [see Dosage and Administration (2.1) ] . Patients receiving ENTYVIO may receive non-live vaccines (e.g., influenza vaccine injection) and may receive live vaccines if the benefits outweigh the risks. There are no data on the secondary transmission of infection by live vaccines in patients receiving ENTYVIO [see Adverse Reactions (6.1) ] .
ADVERSE REACTIONS
The following topics are also discussed in detail in the Warnings and Precautions section:
- Infusion-Related Reactions and Hypersensitivity Reactions [see Warnings and Precautions (5.1) ]
- Infections [see Warnings and Precautions (5.2) ]
- Progressive Multifocal Leukoencephalopathy [see Warnings and Precautions (5.3) ]
- Liver Injury [see Warnings and Precautions (5.4) ]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to intravenous ENTYVIO in 3,326 patients and healthy volunteers in clinical trials, including 1,396 exposed for greater than one year, and 835 exposed for greater than two years.
Intravenous Infusion
The safety data described in Table 2 are derived from four controlled Phase 3 trials (UC Trials I and II and CD Trials I and III); data from adult patients receiving open-label intravenous ENTYVIO treatment at Weeks 0 and 2 (prior to entry into UC Trial II and CD Trial III) and from Weeks 6 to 52 (non-responders at Week 6 of UC Trial I and CD Trial I) are included [see Clinical Studies (14.1 , 14.2) ] .
In these trials, 1,434 patients received ENTYVIO 300 mg intravenously for up to 52 weeks, and 297 patients received placebo for up to 52 weeks. Of these, 769 patients had ulcerative colitis and 962 patients had Crohn's disease. Patients were exposed for a mean duration of 259 days (UC Trials I and II) and 247 days (CD Trials I and III).
Adverse reactions were reported in 52% of patients treated with intravenous ENTYVIO and 45% of patients treated with placebo (UC Trials I and II: 49% with ENTYVIO and 37% with placebo; CD Trials I and III: 55% with ENTYVIO and 47% with placebo). Serious adverse reactions were reported in 7% of patients treated with intravenous ENTYVIO compared to 4% of patients treated with placebo (UC Trials I and II: 8% with ENTYVIO and 7% with placebo; CD Trials I and III: 12% with ENTYVIO and 9% with placebo).
The most common adverse reactions (reported by ≥3% of patients treated with intravenous ENTYVIO in the UC Trials I and II and CD Trials I and III combined group and ≥1% higher than in combined placebo group) were nasopharyngitis, headache, arthralgia, nausea, pyrexia, upper respiratory tract infection, fatigue, cough, bronchitis, influenza, back pain, rash, pruritus, sinusitis, oropharyngeal pain, and pain in extremities (Table 2) .
| Adverse Reaction | ENTYVIO IV Patients who received ENTYVIO for up to 52 weeks. (N=1434) | Placebo Patients who received placebo for up to 52 weeks. (N=297) |
|---|---|---|
| Nasopharyngitis | 13% | 7% |
| Headache | 12% | 11% |
| Arthralgia | 12% | 10% |
| Nausea | 9% | 8% |
| Pyrexia | 9% | 7% |
| Upper respiratory tract infection | 7% | 6% |
| Fatigue | 6% | 3% |
| Cough | 5% | 3% |
| Bronchitis | 4% | 3% |
| Influenza | 4% | 2% |
| Back pain | 4% | 3% |
| Rash | 3% | 2% |
| Pruritus | 3% | 1% |
| Sinusitis | 3% | 1% |
| Oropharyngeal pain | 3% | 1% |
| Pain in extremities | 3% | 1% |
Safety data for patients (n=279) in UC Trials I and II and CD Trials I and III who received intravenous ENTYVIO at Weeks 0 and 2 and were then randomized to placebo at Week 6 for up to 52 weeks, and for patients (n=416) in CD Trial II, a 10-week Crohn's disease trial, are similar to those listed in Table 2 .
Infusion-Related Reactions and Hypersensitivity Reactions
Serious infusion-related reactions and hypersensitivity reactions including anaphylaxis have been reported following intravenous ENTYVIO administration in clinical trials [see Warnings and Precautions (5.1) ] . In UC Trials I and II and CD Trials I and III, one case of anaphylaxis [one out of 1,434 patients treated with intravenous ENTYVIO (0.07%)] was reported by a Crohn's disease patient during the second infusion (symptoms reported were dyspnea, bronchospasm, urticaria, flushing, rash, and increased blood pressure and heart rate) and was managed with discontinuation of infusion and treatment with antihistamine and intravenous hydrocortisone.
In UC Trials I and II and CD Trials I and III, 4% of patients treated with intravenous ENTYVIO and 3% of patients treated with placebo experienced an infusion-related reaction (IRR). The most frequently observed IRRs in the patients treated with intravenous ENTYVIO (reported more than twice) were nausea, headache, pruritus, dizziness, fatigue, infusion-related reaction, pyrexia, urticaria, and vomiting (each of these adverse reactions occurred in <1% in all patients treated with intravenous ENTYVIO) and no individual adverse reaction reported occurred at a rate above 1%. These reactions generally occurred within the first two hours after the infusion and resolved with no treatment or following antihistamine and/or IV hydrocortisone treatment. Less than 1% of patients treated with intravenous ENTYVIO had IRRs assessed by the investigator as severe, and IRRs requiring discontinuation of study treatment occurred in <1%.
In clinical trials, for patients with mild IRRs or hypersensitivity reactions, physicians were allowed to pretreat with standard medical treatment (e.g., antihistamine, hydrocortisone, and/or acetaminophen) prior to next infusion.
Infections
In UC Trials I and II and CD Trials I and III, the rate of infections was 0.85 per patient-year in the patients treated with intravenous ENTYVIO and 0.7 per patient-year in the patients treated with placebo [see Warnings and Precautions (5.2) ] . The infections consisted primarily of nasopharyngitis, upper respiratory tract infection, sinusitis, and urinary tract infection. Two percent of patients discontinued intravenous ENTYVIO due to infections.
In UC Trials I and II and CD Trials I and III, the rate of serious infections was 0.07 per patient-year in patients treated with intravenous ENTYVIO and 0.06 per patient-year in patients treated with placebo. Serious infections were more common in Crohn's disease patients than ulcerative colitis patients, and anal abscesses were the most frequently reported serious adverse reaction in Crohn's disease patients. Over 48 months, there was no increase in the rate of serious infections.
In controlled- and open-label long-term extension trials in adults treated with intravenous ENTYVIO, serious infections have been reported, including anal abscess, sepsis (some fatal), tuberculosis, salmonella sepsis, Listeria meningitis, giardiasis, and cytomegaloviral colitis.
In UC Trials I and II and CD Trials I and III, sepsis, including bacterial sepsis and septic shock, was reported in four of 1,434 (0.3%) patients treated with intravenous ENTYVIO and in two of 297 patients treated with placebo (0.7%). During these trials, two Crohn's disease patients treated with intravenous ENTYVIO died due to reported sepsis or septic shock; both patients had significant comorbidities and a complicated hospital course that contributed to the deaths. In an open label, long-term extension trial, additional cases of sepsis (some fatal), including bacterial sepsis and septic shock, were reported. The rate of sepsis in patients with ulcerative colitis or Crohn's disease receiving intravenous ENTYVIO was two per 1,000 patient-years.
In clinical trials, all patients were screened for tuberculosis. One case of latent, pulmonary tuberculosis was diagnosed during the controlled trials with intravenous ENTYVIO. Additional cases of pulmonary tuberculosis were diagnosed during the open-label trial. All of these observed cases occurred outside the United States (U.S.), and none of the patients had extrapulmonary manifestations.
Liver Injury
There have been reports of elevations of transaminase and/or bilirubin in patients receiving intravenous ENTYVIO [see Warnings and Precautions (5.4) ] . In UC Trials I and II and CD Trials I and III, three patients reported serious adverse reactions of hepatitis, manifested as elevated transaminases with or without elevated bilirubin and symptoms consistent with hepatitis (e.g., malaise, nausea, vomiting, abdominal pain, anorexia). These adverse reactions occurred following two to five intravenous ENTYVIO doses; however, based on case report information it is unclear if the reactions indicated drug-induced or autoimmune etiology. All patients recovered following discontinuation of therapy with some requiring corticosteroid treatment. In controlled trials, the incidence of ALT and AST elevations ≥3× ULN was <2% in patients treated with intravenous ENTYVIO and in patients treated with placebo. In the open-label trial, one additional case of serious hepatitis was observed.
Malignancies
In UC Trials I and II and CD Trials I and III, malignancies (excluding dysplasia and basal cell carcinoma) were reported in six of 1,434 (0.4%) patients treated with intravenous ENTYVIO, including colon cancer (n=2), transitional cell carcinoma (n=1), breast cancer (n=1), carcinoid tumor of the appendix (n=1), and squamous cell carcinoma (n=1). Malignancy was reported in one of 297 (0.3%) patients treated with placebo (squamous cell carcinoma).
Malignancies (excluding dysplasia and basal cell carcinoma) observed during the ongoing open-label long-term extension trial included B-cell lymphoma, breast cancer, colon cancer, malignant hepatic neoplasm, malignant lung neoplasm, malignant melanoma, lung cancer of primary neuroendocrine carcinoma, renal cancer, and squamous cell carcinoma. Overall, the number of malignancies in the clinical trials was small; however, long-term exposure was limited.
Subcutaneous Injection after Two Intravenous Doses of ENTYVIO
ENTYVIO was administered as a subcutaneous injection in adult patients with ulcerative colitis and Crohn’s disease in double-blind, placebo-controlled clinical trials (SC UC Trial and SC CD Trial, respectively). Patients who achieved clinical response following two doses of ENTYVIO administered as an intravenous infusion at Week 0 and Week 2 were randomized 2:1 at Week 6 to ENTYVIO as a subcutaneous injection (N=106) or placebo (N=56) (SC UC Trial) and as a subcutaneous injection (N=275) or placebo (N=134) (SC CD Trial) [see Clinical Studies (14.1 , 14.2) ] .
The safety profile for up to 52 weeks of total treatment was similar between patients who were switched to ENTYVIO as a subcutaneous injection in SC UC and SC CD clinical trials and patients in UC and CD clinical trials who received ENTYVIO as an intravenous infusion (Table 2 ) except for injection site reactions, which were reported with subcutaneous ENTYVIO. Injection site reactions with subcutaneous ENTYVIO were reported in 10% (11/106) of patients in SC UC Trial, including injection site erythema, rash, pruritus, swelling, bruising, and hematoma. Injection site reactions with subcutaneous ENTYVIO were reported in 3% (8/275) of patients in SC CD Trial, including injection site erythema, pruritus, urticaria, pain, rash, and edema.
Live and Oral Vaccines
There are no data on the secondary transmission of infection by live vaccines in patients receiving ENTYVIO.
In a placebo-controlled study of healthy volunteers, 61 subjects were given a single intravenous ENTYVIO 750 mg dose (2.5 times the recommended dose), and 62 subjects received placebo followed by intramuscular vaccination with Hepatitis B surface antigen and oral cholera vaccine. After intramuscular vaccination with three doses of recombinant Hepatitis B surface antigen, those treated with intravenous ENTYVIO did not have lower rates of protective immunity to Hepatitis B virus. However, those exposed to intravenous ENTYVIO did have lower seroconversion rates and anti-cholera titers relative to placebo after receiving the two doses of a killed, oral cholera vaccine. The impact on other oral vaccines and on nasal vaccines in patients is unknown.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of ENTYVIO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune system disorders: Anaphylaxis [see Warnings and Precautions (5.1) ].
Gastrointestinal system disorders: Acute pancreatitis.
Respiratory, thoracic, and mediastinal disorders: Interstitial lung disease, pneumonitis.
DRUG INTERACTIONS
Natalizumab Products
Because of the potential for increased risk of PML and other infections, avoid the concomitant use of ENTYVIO with natalizumab products.
TNF Blockers
Because of the potential for increased risk of infections, avoid the concomitant use of ENTYVIO with TNF blockers.
CYP450 Substrates
The formation of CYP450 enzymes may be suppressed by increased levels of certain cytokines (e.g., IL-6, IL-10, TNFα, IFN) during chronic inflammation. Therefore, use of ENTYVIO may normalize the formation of CYP450 enzymes by modulating the underlying disease. Upon initiation or discontinuation of ENTYVIO in patients treated with CYP450 substrates, monitor drug concentrations or other therapeutic parameters, and adjust the dosage of the CYP substrate as needed. See the prescribing information of specific CYP substrates.
DESCRIPTION
Vedolizumab, an integrin receptor antagonist, is a humanized IgG 1 monoclonal antibody produced in Chinese hamster ovary cells that binds to the human α4β7 integrin. ENTYVIO has an approximate molecular weight of 147 kilodaltons.
Intravenous ENTYVIO
ENTYVIO (vedolizumab) for injection is supplied as a sterile, white to off-white, preservative-free, lyophilized cake for intravenous infusion. After reconstitution with 4.8 mL Sterile Water for Injection, USP, 0.9% Sodium Chloride Injection, USP, or Lactated Ringer's Injection, USP, the resulting concentration is 60 mg/mL with a deliverable volume of 5 mL (300 mg) and the resulting pH is approximately 6.3.
Each single-dose vial contains 300 mg vedolizumab, arginine hydrochloride (131.7 mg), histidine (23 mg), histidine monohydrochloride (21.4 mg), polysorbate 80 (3 mg), and sucrose (500 mg).
Subcutaneous ENTYVIO
ENTYVIO (vedolizumab) injection is supplied as a sterile, clear to moderately opalescent, colorless to slightly yellow, preservative-free solution for subcutaneous administration.
Each single-dose prefilled syringe or single-dose prefilled pen (ENTYVIO PEN) contains 108 mg vedolizumab, arginine hydrochloride (17.77 mg), citric acid monohydrate (0.18 mg), histidine (3.86 mg), histidine monohydrochloride (1.86 mg), polysorbate 80 (1.35 mg), sodium citrate dihydrate (4.71 mg) and Sterile Water for Injection, USP, at a pH of 6.5.
CLINICAL PHARMACOLOGY
Mechanism of Action
Vedolizumab is a humanized monoclonal antibody that specifically binds to the α4β7 integrin and blocks the interaction of α4β7 integrin with mucosal addressin cell adhesion molecule-1 (MAdCAM-1) and inhibits the migration of memory T-lymphocytes across the endothelium into inflamed gastrointestinal parenchymal tissue. Vedolizumab does not bind to or inhibit function of the α4β1 and αEβ7 integrins and does not antagonize the interaction of α4 integrins with vascular cell adhesion molecule-1 (VCAM-1).
The α4β7 integrin is expressed on the surface of a discrete subset of memory T-lymphocytes that preferentially migrate into the gastrointestinal tract. MAdCAM-1 is mainly expressed on gut endothelial cells and plays a critical role in the homing of T-lymphocytes to gut lymph tissue. The interaction of the α4β7 integrin with MAdCAM-1 has been implicated as an important contributor to the chronic inflammation that is a hallmark of ulcerative colitis and Crohn's disease.
Pharmacodynamics
In clinical trials with intravenous ENTYVIO at doses ranging from 0.2 to 10 mg/kg (which includes doses outside of the recommended dose), saturation of α4β7 receptors on subsets of circulating lymphocytes involved in gut-immune surveillance was observed.
In clinical trials with intravenous ENTYVIO at doses ranging from 0.2 to 10 mg/kg and 180 to 750 mg (which include doses outside of the recommended dose) in healthy subjects and in patients with ulcerative colitis or Crohn's disease, vedolizumab did not elevate neutrophils, basophils, eosinophils, B-helper and cytotoxic T-lymphocytes, total memory helper T-lymphocytes, monocytes or natural killer cells.
A reduction in gastrointestinal inflammation was observed in rectal biopsy specimens from Phase 2 ulcerative colitis patients exposed to ENTYVIO for four or six weeks compared to placebo control as assessed by histopathology.
In a study of 14 healthy subjects, ENTYVIO did not affect the CD4+ lymphocyte cell counts, CD8+ lymphocyte cell counts, or the CD4+:CD8+ ratios in the CSF [see Clinical Pharmacology (12.3) ] .
Pharmacokinetics
Similar pharmacokinetics were observed in ulcerative colitis and Crohn's disease patients administered 300 mg ENTYVIO as a 30-minute intravenous infusion on Weeks 0, 2, and 6, and then every eight weeks up to Week 52 (Table 3) .
| Patient Population | Weeks 0, 2, and 6 ENTYVIO 300 mg Intravenously | After Week 6 to 52 ENTYVIO 300 mg Intravenously Every 8 Weeks |
|---|---|---|
| Trough Serum Concentration at Week 6 (mcg/mL) | Trough Serum Concentration at Week 46 Steady-state trough serum concentration. (mcg/mL) | |
| Ulcerative Colitis | 26.3 ± 12.9 (N=210) | 11.2 ± 7.2 (N=77) |
| Crohn's Disease | 27.4 ± 19.2 (N=198) | 13.0 ± 9.1 (N=72) |
In ulcerative colitis and Crohn’s disease patients, administered 300 mg ENTYVIO as a 30-minute intravenous infusion on Weeks 0 and 2, followed by 108 mg ENTYVIO as a subcutaneous injection every 2 weeks starting from Week 6, the mean steady state serum trough concentrations were 35.8 mcg/mL (SD ± 15.2) and 31.4 mcg/mL (SD ± 14.7), respectively.
The bioavailability of vedolizumab following a 108 mg single-dose subcutaneous injection relative to a 300 mg single-dose intravenous infusion in healthy subjects was approximately 75%. Following a 108 mg single-dose subcutaneous injection in healthy subjects, the median T max was 7 days with a range of 3 to 14 days and the mean C max was 15.4 mcg/mL (SD ± 3.2).
Vedolizumab clearance depends on both linear and nonlinear pathways; the nonlinear clearance decreases with increasing concentrations. Population pharmacokinetic analyses indicated that the linear clearance was approximately 0.16 L/day, the serum half-life was approximately 26 days, and the distribution volume was approximately 5 L.
Vedolizumab was not detected in the cerebrospinal fluid (CSF) of 14 healthy subjects at five weeks after a single intravenous administration of 450 mg ENTYVIO (1.5 times the recommended dosage).
Specific Populations
Population pharmacokinetic analysis showed that the severity of disease state, body weight, prior treatment with TNF blocker therapy, age (18 to 78 years), serum albumin, coadministered immunomodulators (including azathioprine, 6-mercaptopurine, methotrexate), and coadministered aminosalicylates did not have a clinically meaningful effect on the pharmacokinetics of ENTYVIO.
Pharmacokinetics of vedolizumab in patients with renal or hepatic insufficiency have not been studied.
Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of ENTYVIO or of other vedolizumab products.
Adults Treated with Intravenous ENTYVIO
The incidence of anti-drug antibodies to intravenous ENTYVIO using a drug-tolerant electrochemiluminescence (ECL) method for patients in UC Trials I and II and CD Trials I and III who had continuous ENTYVIO treatment administered as an intravenous infusion for 52 weeks was 6% (86 out of 1,427 total ENTYVIO-treated patients). Of the 86 patients who tested positive for anti-vedolizumab antibodies, 20 patients were persistently positive (at two or more consecutive study visits) and 56 developed neutralizing antibodies to vedolizumab.
Among the ENTYVIO-treated patients who developed persistent anti-vedolizumab antibodies, 14/20 patients had serum vedolizumab trough concentrations that were markedly reduced or undetectable and 15/20 patients did not achieve clinical remission at Week 52 in UC Trials I and II and CD Trials I and III. Because of the low occurrence of persistent anti-vedolizumab antibodies (1%; 20/1,427), the effect of these antibodies on the safety and effectiveness of ENTYVIO in these studies has not been fully characterized.
Adults Treated with Subcutaneous ENTYVIO
The incidence of anti-drug antibodies to ENTYVIO using a drug-tolerant ECL method for patients in SC UC Trial and SC CD Trial who had continuous treatment for 52 weeks was 3.4% (13 out of 381 total patients treated with subcutaneous ENTYVIO). Of the 13 patients who tested positive for anti-vedolizumab antibodies, 7 patients were persistently positive (at two or more consecutive study visits) and 7 patients developed neutralizing antibodies to vedolizumab. Two of the 7 patients with Crohn’s disease and none of the 6 patients with ulcerative colitis who had positive anti-vedolizumab antibodies achieved clinical remission at Week 52. There is insufficient data to assess the effect of anti-drug antibodies on pharmacokinetics, effectiveness, and safety of ENTYVIO in the SC UC and SC CD trials.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of vedolizumab. Studies to evaluate the possible impairment of fertility or mutagenic potential of vedolizumab have not been performed.
CLINICAL STUDIES
Clinical Studies in Ulcerative Colitis
Intravenous Administration
The safety and efficacy of intravenous ENTYVIO were evaluated in two randomized, double-blind, placebo-controlled trials (UC Trials I and II) in adult patients with moderately to severely active ulcerative colitis (UC) defined as Mayo score of 6 to 12 with endoscopy subscore of two or three. The Mayo score ranges from 0 to 12 and has four subscales that are each scored from zero (normal) to three (most severe): stool frequency, rectal bleeding, findings on endoscopy, and physician global assessment. An endoscopy subscore of two is defined by marked erythema, lack of vascular pattern, friability, and erosions; an endoscopy subscore of three is defined by spontaneous bleeding and ulceration.
Enrolled patients in the U.S. had over the previous five-year period an inadequate response or intolerance to immunomodulator therapy (i.e., azathioprine or 6-mercaptopurine) and/or an inadequate response, loss of response, or intolerance to a TNF blocker. Outside the U.S., prior treatment with corticosteroids was sufficient for entry if over the previous five-year period the patients were corticosteroid dependent (i.e., unable to successfully taper corticosteroids without a return of the symptoms of UC) or had an inadequate response or intolerance to corticosteroids.
Patients that had received natalizumab ever in the past, and patients that had received a TNF blocker in the past 60 days were excluded from enrollment. Concomitant use of natalizumab or a TNF blocker was not allowed.
UC Trial I - Intravenous
In UC Trial I, 374 patients were randomized in a double-blind fashion (3:2) to receive ENTYVIO 300 mg or placebo by intravenous infusion at Week 0 and Week 2. Efficacy assessments were at Week 6. Concomitant stable dosages of aminosalicylates, corticosteroids (prednisone dosage ≤30 mg/day or equivalent), and immunomodulators (azathioprine or 6-mercaptopurine) were permitted through Week 6.
At baseline, patients received corticosteroids (54%), immunomodulators (azathioprine or 6-mercaptopurine) (30%), and/or aminosalicylates (74%). Thirty-nine percent of patients had an inadequate response, loss of response, or intolerance to a TNF blocker therapy. Eighteen percent of patients had an inadequate response, inability to taper or intolerance to prior corticosteroid treatment only (i.e., had not received prior immunomodulators or TNF blockers). The median baseline Mayo score was 9 in the ENTYVIO group and 8 in the placebo group.
In UC Trial I, a greater percentage of patients treated with intravenous ENTYVIO compared to patients treated with placebo achieved clinical response at Week 6 (defined in Table 4 ). A greater percentage of patients treated with intravenous ENTYVIO compared to patients treated with placebo also achieved clinical remission at Week 6 (defined in Table 4 ). In addition, a greater percentage of patients treated with ENTYVIO had improvement of endoscopic appearance of the mucosa at Week 6 (defined in Table 4 ).
| Endpoint | Placebo N=149 | ENTYVIO IV N=225 | p-value | Treatment Difference and 95% CI |
|---|---|---|---|---|
| Clinical Response Clinical response: reduction in complete Mayo score of ≥3 points and ≥30% from baseline with an accompanying decrease in rectal bleeding subscore of ≥1 point or absolute rectal bleeding subscore of ≤1 point. at Week 6 | 26% | 47% | <0.001 | 22% (12%, 32%) |
| Clinical Remission Clinical remission: complete Mayo score of ≤2 points and no individual subscore >1 point. at Week 6 | 5% | 17% | 0.001 | 12% (5%, 18%) |
| Improvement of Endoscopic Appearance of the Mucosa Improvement of endoscopic appearance of the mucosa: Mayo endoscopy subscore of 0 (normal or inactive disease) or 1 (erythema, decreased vascular pattern, mild friability). at Week 6 | 25% | 41% | 0.001 | 16% (6%, 26%) |
UC Trial II - Intravenous
In order to be randomized to treatment in UC Trial II, patients had to have received intravenous ENTYVIO and be in clinical response at Week 6. Patients could have come from either UC Trial I or from a group who received ENTYVIO open-label.
In UC Trial II, 373 patients were randomized in a double-blind fashion (1:1:1) to one of the following regimens beginning at Week 6: intravenous ENTYVIO 300 mg every eight weeks, intravenous ENTYVIO 300 mg every four weeks or placebo every four weeks. Efficacy assessments were at Week 52. Concomitant aminosalicylates and corticosteroids were permitted through Week 52. Concomitant immunomodulators (azathioprine or 6-mercaptopurine) were permitted outside the U.S. but were not permitted beyond Week 6 in the U.S.
At Week 6, patients were receiving corticosteroids (61%), immunomodulators (azathioprine or 6-mercaptopurine) (32%), and aminosalicylates (75%). Thirty-two percent of patients had an inadequate response, loss of response or intolerance to a TNF blocker therapy. At Week 6, the median Mayo score was 8 in the ENTYVIO every eight week group, the ENTYVIO every four week group, and the placebo group. Patients who had achieved clinical response at Week 6 and were receiving corticosteroids were required to begin a corticosteroid-tapering regimen at Week 6.
In UC Trial II, a greater percentage of patients in groups treated with intravenous ENTYVIO as compared to placebo achieved clinical remission at Week 52, and maintained clinical response (clinical response at both Weeks 6 and 52) (Table 5) . In addition, a greater percentage of patients in groups treated with intravenous ENTYVIO as compared to placebo were in clinical remission at both Weeks 6 and 52, and had improvement of endoscopic appearance of the mucosa at Week 52 (Table 5) . In the subgroup of patients who achieved clinical response at Week 6 and were receiving corticosteroid medication at baseline, a greater proportion of patients in groups treated with intravenous ENTYVIO as compared to placebo discontinued corticosteroids and were in clinical remission at Week 52 (Table 5) .
The ENTYVIO every four week dosing regimen did not demonstrate additional clinical benefit over the every eight dosing week regimen. The every four week dosing regimen is not the recommended dosing regimen [see Dosage and Administration (2.2) ] .
| Endpoint | Placebo The placebo group includes those patients who received ENTYVIO at Week 0 and Week 2 and were randomized to receive placebo from Week 6 through Week 52. N=126 | ENTYVIO IV Every 8 Weeks N=122 | p-value | Treatment Difference and 95% CI |
|---|---|---|---|---|
| Clinical Remission at Week 52 | 16% | 42% | <0.001 | 26% (15%, 37%) |
| Clinical Response at both Weeks 6 and 52 | 24% | 57% | <0.001 | 33% (21%, 45%) |
| Improvement of Endoscopic Appearance of the Mucosa Improvement of endoscopic appearance of the mucosa: Mayo endoscopy subscore of 0 (normal or inactive disease) or 1 (erythema, decreased vascular pattern, mild friability) at Week 52. at Week 52 | 20% | 52% | <0.001 | 32% (20%, 44%) |
| Clinical Remission at both Weeks 6 and 52 | 9% | 21% | 0.008 | 12% (3%, 21%) |
| Corticosteroid-free Clinical Remission Corticosteroid-free clinical remission: Assessed in the subgroup of patients who were receiving corticosteroids at baseline and who were in clinical response at Week 6 (n=72 for placebo and n=70 for ENTYVIO every eight weeks). Corticosteroid-free clinical remission was defined as the proportion of patients in this subgroup that discontinued corticosteroids by Week 52 and were in clinical remission at Week 52. | 14% | 31% | 0.012 | 18% (4%, 31%) |
Subcutaneous Administration
SC UC Trial - Subcutaneous
The safety and efficacy of subcutaneous ENTYVIO was evaluated in a randomized, double-blind, placebo-controlled trial (SC UC Trial; NCT02611830) in adult patients with moderately to severely active ulcerative colitis defined as Mayo score of 6 to 12 with endoscopy subscore of two or three. The baseline Mayo score was between 9 to 12 in about 62% and six to eight in about 38% of the overall trial population.
The trial included patients who had experienced an inadequate response to, loss of response to, or intolerance to at least one of the following: at least one 12-week regimen of azathioprine or 6-mercaptopurine, induction with a TNF blocker, or corticosteroids. Patients were permitted to use concomitant stable doses of oral aminosalicylates, oral corticosteroids (prednisone ≤30 mg/day or budesonide ≤9 mg/day), azathioprine or 6-mercaptopurine, probiotics and/or antidiarrheals. Concomitant biologic therapies, rectal treatment with 5-aminosalicylic acid or corticosteroid enemas/suppositories were prohibited.
All patients received open-label intravenous ENTYVIO 300 mg at Week 0 and Week 2. In order to be randomized to treatment in SC UC Trial, patients had to be in clinical response at Week 6. A total of 162 patients were randomized at Week 6 in a double-blind fashion (2:1) to ENTYVIO 108 mg administered by subcutaneous injection or placebo every 2 weeks. Efficacy assessments were at Week 52.
Beginning at Week 6, patients who were receiving corticosteroids were required to begin a corticosteroid tapering regimen.
At the time of randomization into the double-blind phase (Week 6), patients were receiving corticosteroids (51%), immunomodulators (azathioprine or 6-mercaptopurine) (33%), and aminosalicylates (80%). Thirty-seven percent of patients had an inadequate response, loss of response, or intolerance to a TNF blocker therapy prior to enrollment.
Patients in the double-blind phase had a mean age of 39 years (range 18 to 69 years); 61% were male; 83% identified as White, 17% as Asian, and <1% identified as another racial group.
The primary endpoint was the proportion of patients in clinical remission defined as a Mayo score of ≤2 points and no individual subscore >1 point at Week 52. Secondary endpoints included the proportion of patients with improvement of endoscopic appearance of the mucosa at Week 52 and clinical response at both Weeks 6 and 52 (see Table 6 ) .
| Endpoint | Placebo The placebo group includes those subjects who received intravenous vedolizumab at Week 0 and Week 2 and were randomized to receive placebo from Week 6 through Week 52. | ENTYVIO 108 mg SC Every 2 Weeks Starting at Week 6 following two intravenous doses of ENTYVIO 300 mg administered as an intravenous infusion at Weeks 0 and 2. | Estimate Estimated treatment difference is based on the Cochran-Mantel-Haenszel method. of Treatment Difference vs. Placebo (95% CI) |
|---|---|---|---|
| Clinical Remission Clinical remission: Complete Mayo score of ≤2 points and no individual subscore >1 point at Week 52. at Week 52 | |||
| Total Population | N=56 14% | N=106 46% | 32 (20, 45) p <0.001 |
| Prior TNF blocker failure | N=20 10% | N=40 35% | |
| Without prior TNF blocker failure | N=36 17% | N=66 53% | |
| Improvement of Endoscopic Appearance of the Mucosa at Week 52 Improvement of endoscopic appearance of the mucosa: Mayo endoscopic subscore of ≤1 point. | |||
| Total Population | N=56 21% | N=106 57% | 36 (22, 49) |
| Prior TNF blocker failure | N=20 10% | N=40 48% | |
| Without prior TNF blocker failure | N=36 28% | N=66 62% | |
| Clinical Response at both Weeks 6 and 52 Clinical response: reduction in complete Mayo score of ≥3 points and ≥30% from baseline with an accompanying decrease in rectal bleeding subscore of ≥1 point or absolute rectal bleeding subscore of ≤1 point. | |||
| Total Population | N=56 29% | N=106 64% | 36 (21, 51) |
| Prior TNF blocker failure | N=20 20% | N=40 68% | |
| Without prior TNF blocker failure | N=36 33% | N=66 62% | |
Maintenance of remission at Week 52 in the subgroup of patients who were in remission at Week 6, was 64% (16/25) in the ENTYVIO-treated group compared to 20% (3/15) in the placebo group. The treatment difference was 44% (95% CI: 9%, 69%).
Clinical Studies in Crohn's Disease
Intravenous Administration
The safety and efficacy of intravenous ENTYVIO were evaluated in three randomized, double-blind, placebo-controlled clinical trials (CD Trials I, II, and III) in adult patients with moderately to severely active Crohn's disease (CD) (Crohn's Disease Activity Index [CDAI] score of 220 to 450).
Enrolled patients in the U.S. had over the previous five-year period an inadequate response or intolerance to immunomodulator therapy (i.e., azathioprine, 6-mercaptopurine, or methotrexate) and/or an inadequate response, loss of response, or intolerance to one or more TNF blockers. Outside the U.S., prior treatment with corticosteroids was sufficient for entry if over the previous five-year period the patients were corticosteroid dependent (i.e., unable to successfully taper corticosteroids without a return of the symptoms of CD) or had an inadequate response or intolerance to corticosteroids.
Patients that had received natalizumab ever in the past, and patients that had received a TNF blocker in the past 30 to 60 days were excluded from enrollment. Concomitant use of natalizumab or a TNF blocker was not allowed.
CD Trial I - Intravenous
In CD Trial I, 368 patients were randomized in a double-blind fashion (3:2) to receive ENTYVIO 300 mg or placebo by intravenous infusion at Week 0 and Week 2. Efficacy assessments were at Week 6. Concomitant stable dosages of aminosalicylates, corticosteroids (prednisone dosage ≤30 mg/day or equivalent), and immunomodulators (azathioprine, 6-mercaptopurine or methotrexate) were permitted through Week 6.
At baseline, patients were receiving corticosteroids (49%), immunomodulators (azathioprine, 6-mercaptopurine, or methotrexate) (35%), and/or aminosalicylates (46%). Forty-eight percent of the patients had an inadequate response, loss of response, or intolerance to a TNF blocker therapy. Seventeen percent of patients had inadequate response, inability to taper, or intolerance to prior corticosteroid treatment only (i.e., had not received prior immunomodulators or TNF blockers). The median baseline CDAI score was 324 in the intravenous ENTYVIO group and 319 in the placebo group.
In CD Trial I, a statistically significantly higher percentage of patients treated with intravenous ENTYVIO achieved clinical remission (defined as CDAI ≤150) as compared to placebo at Week 6 (Table 7) . The difference in the percentage of patients who demonstrated clinical response (defined as a ≥100-point decrease in CDAI score from baseline), was however, not statistically significant at Week 6.
CD Trial II - Intravenous
Compared to CD Trial I, CD Trial II enrolled a higher number of patients who had over the previous five-year period had an inadequate response, loss of response, or intolerance to one or more TNF blockers (76%); this was the primary analysis population. In CD Trial II, 416 patients were randomized in a double-blind fashion (1:1) to receive either intravenous ENTYVIO 300 mg or placebo at Weeks 0, 2, and 6. Efficacy assessments were at Weeks 6 and 10. Concomitant aminosalicylates, corticosteroids, and immunomodulators (azathioprine, 6-mercaptopurine, or methotrexate) were permitted through Week 10.
At baseline, patients were receiving corticosteroids (54%), immunomodulators (azathioprine, 6-mercaptopurine, or methotrexate) (34%), and aminosalicylates (31%). The median baseline CDAI score was 317 in the ENTYVIO group and 301 in the placebo group.
For the primary endpoint (clinical remission at Week 6), treatment with intravenous ENTYVIO did not result in statistically significant improvement over placebo ( Table 7 ) . Secondary endpoints including assessments at Week 10 were not tested because the primary endpoint was not statistically significant.
| Endpoint | Placebo | ENTYVIO IV | p-value | Treatment Difference and 95% CI |
|---|---|---|---|---|
| CD Trial I: Clinical Remission Clinical Remission: CDAI ≤150. at Week 6 | 7% (10/148) | 15% (32/220) | 0.041 Adjusted p-value for multiple comparisons of two primary endpoints. | 8% (1%, 14%) |
| CD Trial II The primary analysis population for CD Trial II was patients that had an inadequate response, loss of response, or intolerance to one or more TNF blockers (76% of the overall population). : Clinical Remissionat Week 6 | 12% (19/157) | 15% (24/158) | NS NS: Not significant (Secondary endpoints including assessments at Week 10 were not tested because the CD Trial II primary endpoint was not statistically significant). | 3% (-5%, 11%) |
CD Trial III - Intravenous
In order to be randomized to treatment in CD Trial III, patients had to have received intravenous ENTYVIO and be in clinical response (defined as a ≥70-point decrease in CDAI score from baseline) at Week 6. Patients could have come from either CD Trial I or from a group who received intravenous ENTYVIO open-label.
In CD Trial III, 461 patients were randomized in a double-blind fashion (1:1:1) to one of the following regimens beginning at Week 6: intravenous ENTYVIO 300 mg every eight weeks, intravenous ENTYVIO 300 mg every four weeks or placebo every four weeks. Efficacy assessments were at Week 52. Concomitant aminosalicylates and corticosteroids were permitted through Week 52. Concomitant immunomodulators (azathioprine, 6-mercaptopurine, or methotrexate) were permitted outside the U.S. but were not permitted beyond Week 6 in the U.S.
At Week 6, patients were receiving corticosteroids (59%), immunomodulators (azathioprine, 6-mercaptopurine, or methotrexate) (31%), and aminosalicylates (41%). Fifty-one percent of patients had an inadequate response, loss of response, or intolerance to a TNF blocker therapy. At Week 6, the median CDAI score was 322 in the intravenous ENTYVIO every eight week group, 316 in the intravenous ENTYVIO every four week group, and 315 in the placebo group. Patients who had achieved clinical response (≥70 decrease in CDAI score from baseline) at Week 6 and were receiving corticosteroids were required to begin a corticosteroid-tapering regimen at Week 6.
In CD Trial III a greater percentage of patients in groups treated with intravenous ENTYVIO as compared to placebo were in clinical remission (defined as CDAI score ≤150) at Week 52. A greater percentage of patients in groups treated with intravenous ENTYVIO as compared to placebo had a clinical response (defined as ≥100 decrease in CDAI score from baseline) at Week 52 (Table 8) . In the subgroup of patients who were receiving corticosteroids at baseline and who were in clinical response at Week 6 (defined as ≥70 decrease in CDAI score from baseline), a greater proportion of patients in groups treated with intravenous ENTYVIO as compared to placebo discontinued corticosteroids by Week 52 and were in clinical remission at Week 52 (Table 8) .
The ENTYVIO every four week dosing regimen did not demonstrate additional clinical benefit over the every eight dosing week regimen. The every four week dosing regimen is not the recommended dosing regimen [see Dosage and Administration (2.2) ] .
| Endpoint | Placebo The placebo group includes those patients who received ENTYVIO at Week 0 and Week 2, and were randomized to receive placebo from Week 6 through Week 52. N=153 | ENTYVIO IV Every 8 Weeks N=154 | p-value | Treatment Difference and 95% CI |
|---|---|---|---|---|
| Clinical Remission Clinical remission: CDAI ≤150. at Week 52 | 22% | 39% | 0.001 | 17% (7%, 28%) |
| Clinical Response Clinical response: ≥100 decrease in CDAI from baseline. at Week 52 | 30% | 44% | 0.013 | 13% (3%, 24%) |
| Corticosteroid-free Clinical Remission Corticosteroid-free clinical remission: Assessed in the subgroup of patients who were receiving corticosteroids at baseline and who were in clinical response (defined as ≥70 decrease in CDAI from baseline) at Week 6 (n=82 for placebo and n=82 for ENTYVIO every eight weeks). Corticosteroid-free clinical remission was defined as the proportion of patients in this subgroup that discontinued corticosteroids by Week 52 and were in clinical remission at Week 52. | 16% | 32% | 0.015 | 16% (3%, 29%) |
Subcutaneous Administration
SC CD Trial - Subcutaneous
The safety and efficacy of subcutaneous ENTYVIO was evaluated in a randomized, double-blind, placebo-controlled trial (SC CD Trial; NCT02611817) in adult patients with moderately to severely active Crohn’s disease defined as CDAI score of 220 to 450. At baseline, the median CDAI score was 316 (range: 198 to 559).
The trial included patients who had experienced an inadequate response to, loss of response to, or intolerance to at least one of the following: corticosteroids, immunomodulators (azathioprine, 6-mercaptopurine, or methotrexate), or TNF blockers (including primary non-responders). Patients were permitted to use concomitant stable doses of oral aminosalicylates, oral corticosteroids (prednisone ≤30 mg/day, budesonide ≤9 mg/day, or equivalent steroid), immunomodulators, probiotics, antidiarrheals, and/or antibiotics. Concomitant biologic therapies, rectal treatment with 5-aminosalicylic acid or corticosteroid enemas/suppositories were prohibited.
All patients received open-label intravenous ENTYVIO 300 mg at Week 0 and Week 2. In order to be randomized to treatment in SC CD Trial, patients had to be in clinical response (defined as a ≥70-point decrease in the CDAI score from baseline) at Week 6. A total of 409 patients were randomized at Week 6 in a double-blind fashion (2:1) to ENTYVIO 108 mg administered by subcutaneous injection or placebo every 2 weeks. Efficacy assessments were at Week 52.
Beginning at Week 6, patients who were receiving corticosteroids were required to begin a corticosteroid tapering regimen.
At the time of randomization into the double-blind phase (Week 6), patients were receiving corticosteroids (45%), immunomodulators (32%), and aminosalicylates (45%). Fifty-one percent of patients had an inadequate response, loss of response, or intolerance to a TNF blocker therapy prior to enrollment.
Patients in the double-blind phase had a mean age of 38 years (range 18 to 76 years); 55% were male; 91% identified as White, 6% as Asian, and 3% identified as another racial group.
The primary endpoint was the proportion of patients with clinical remission (CDAI score ≤150) at Week 52 (see Table 9 ) .
| Endpoint | Placebo The placebo group includes those subjects who received intravenous vedolizumab at Week 0 and Week 2, and were randomized to receive placebo from Week 6 through Week 52. | ENTYVIO SC 108 mg Every 2 Weeks | Estimate Estimate of treatment difference and the p-value are based on the Cochran-Mantel-Haenszel method. of Treatment Difference (95% CI) Vedolizumab SC vs. Placebo |
|---|---|---|---|
| Clinical Remission Clinical remission: CDAI score ≤150, at Week 52. at Week 52 | |||
| Total Population | N=134 34% | N=275 48% | 14 (4, 24) p <0.01 |
| Prior TNF blocker failure /exposure | N=71 27% | N=168 48% | |
| Without prior TNF blocker failure /exposure | N=63 43% | N=107 49% | |
Among patients using oral corticosteroids at baseline (Week 0) and achieving clinical response at Week 6, 45% (43/95) treated with subcutaneous ENTYVIO compared to 18% (8/44) treated with placebo discontinued corticosteroids and were in clinical remission at Week 52. This result was not statistically significant under the prespecified multiple testing procedure.
HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Intravenous Infusion
ENTYVIO ® (vedolizumab) for injection for intravenous infusion is supplied in sterile single-dose glass vials, containing 300 mg of vedolizumab as a white to off-white lyophilized cake.
- ENTYVIO: 300 mg single-dose vial in individual carton: NDC 64764-300-20
Subcutaneous Injection
ENTYVIO (vedolizumab) injection for subcutaneous use is available in a prefilled syringe or a prefilled pen as a clear to moderately opalescent and colorless to slightly yellow solution.
The single-dose, disposable ENTYVIO prefilled syringe and single-dose, disposable ENTYVIO prefilled pen (ENTYVIO PEN) are comprised of a 1 mL long glass syringe with a fixed 27 gauge thin wall, ½ inch needle. The syringe has a rubber needle cover encased in a plastic shell and rubber stopper. Not made with natural rubber latex.
- ENTYVIO: 108 mg/0.68 single-dose prefilled syringe in an individual carton: NDC 64764-107-11
- ENTYVIO PEN : 108 mg/0.68 single-dose prefilled pen in an individual carton: NDC 64764-108-21
Storage and Handling
- Refrigerate ENTYVIO unopened vials, prefilled syringes, and prefilled pens at 2°C to 8°C (36° to 46°F).
- If needed, the ENTYVIO prefilled syringe or ENTYVIO PEN can be left out of the refrigerator in the original package at room temperature up to 25°C (77°F) for up to 7 days (for example, when traveling). Do not use ENTYVIO prefilled syringe or ENTYVIO PEN if left out of the refrigerator for more than 7 days.
- Do not freeze ENTYVIO vial, prefilled syringe, or prefilled pen. Do not use ENTYVIO vial, prefilled syringe, or prefilled pen if it has been frozen.
- Do not shake the ENTYVIO prefilled syringe or ENTYVIO PEN.
- Retain in original package to protect from light until the time of use.
INSTRUCTIONS FOR USE
ENTYVIO ® (en ti' vee oh) PEN (vedolizumab) injection, for subcutaneous use Single-dose prefilled pen
This Instructions for Use contains information on how to inject ENTYVIO.
Your ENTYVIO single-dose prefilled pen
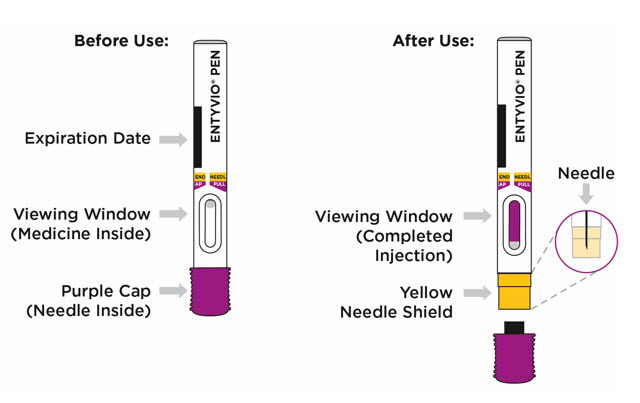
Important information you need to know before injecting ENTYVIO:
|
Storing ENTYVIO
- Store your prefilled pen in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Your prefilled pen can be left in its box at room temperature up to 77°F (25°C) for up to 7 days (for example, when traveling). Do not use the prefilled pen if it is left out of the refrigerator for more than 7 days.
- Do not freeze the prefilled pen.
- Do not leave the prefilled pen in direct sunlight.
- Throw away the prefilled pen in a FDA-cleared sharps disposal container if it has been left out of the refrigerator for more than 7 days, frozen, or left in direct sunlight. See Step 14 for instructions on how to throw away (dispose of) the prefilled pen.
- Always keep ENTYVIO PENs, the sharps disposal container, and all medicines out of the reach of children.
| U.S. License No. 1898 | ||
| Getting Your Supplies Ready | ||
| Step 1. Remove the ENTYVIO PEN box from the refrigerator | ||
Take 1 prefilled pen box from the refrigerator and check the expiration date on the box (see Figure A ).
| 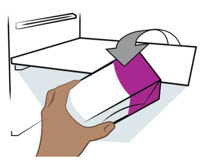 Figure A Figure A | |
| Step 2. Wait 30 minutes | ||
Wait 30 minutes and let the prefilled pen come to room temperature (see Figure B ).
|  Figure B Figure B | |
| Step 3. Gather supplies | ||
Find a clean, flat surface like a table. Gather supplies that are not in the prefilled pen box (see Figure C ).
|  Figure C Figure C | |
| Preparing to Inject ENTYVIO | ||
| Step 4. Wash hands | ||
| Wash your hands with soap and clean water (see Figure D ). | 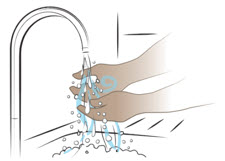 Figure D Figure D | |
| Step 5. Remove the prefilled pen from the tray | ||
Peel off the paper on the tray and lift the prefilled pen straight out (see Figure E ).
| 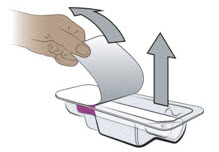 Figure E Figure E | |
| Step 6. Inspect the prefilled pen | ||
Check the expiration date (EXP) printed on the prefilled pen and the medicine in the prefilled pen viewing window (see Figure F ). The medicine should be colorless to pale yellow. It is normal to see air bubbles. Inspect the prefilled pen for any damage.
| 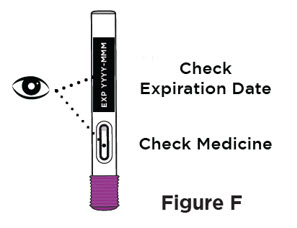 | |
| Step 7. Choose injection site | ||
Choose an injection site on your bare skin from one of the following (see Figure G ):
| 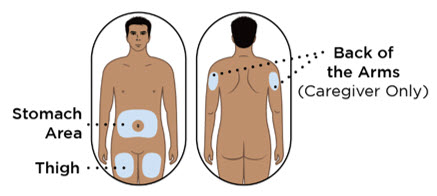 Figure G Figure G | |
| Step 8. Clean the injection site | ||
Clean the injection site with an alcohol pad (see Figure H ). Let your skin dry.
| 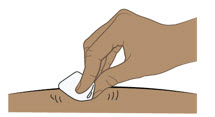 Figure H Figure H | |
| Continue to Step 9 ➔ | ||
| Injecting ENTYVIO | ||
| Step 9. Remove the purple cap and throw it away | ||
When you are ready to inject, pull the purple cap straight off and throw it right away in the sharps disposal container (see Figure I ).
| 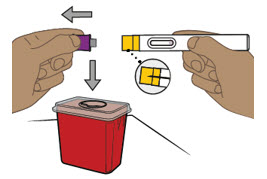 Figure I Figure I | |
| Step 10. Place the prefilled pen on the injection site | ||
| 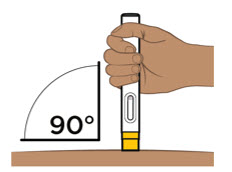 Figure J Figure J | |
| Step 11. Start injecting ENTYVIO | Step 12. Complete injecting ENTYVIO | |
Push the prefilled pen straight down and hold for at least 10 seconds (see Figure K ) .
| Continue holding the prefilled pen with constant pressure until the viewing window has filled with purple to make sure you have received your full dose (see Figure L ).
| |
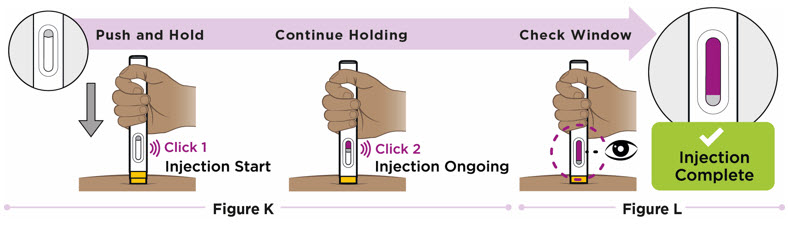 | ||
| Step 13. Lift prefilled pen from skin | ||
Lift the prefilled pen from the injection site (see Figure M ). The yellow needle shield will drop down and lock over the needle.
| 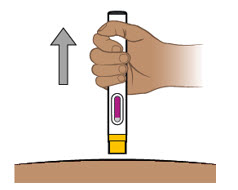 Figure M Figure M | |
| Step 14. Throw away (dispose of) the prefilled pen | ||
Throw away (dispose of) the used prefilled pen in a FDA-cleared sharps disposal container right away after use (see Figure N ). Do not recycle or throw away the prefilled pen in your household trash.
| 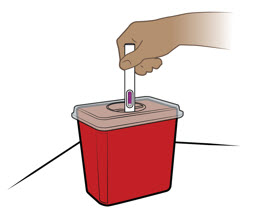 Figure N Figure N | |
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Revised: 5/2024
Mechanism of Action
Vedolizumab is a humanized monoclonal antibody that specifically binds to the α4β7 integrin and blocks the interaction of α4β7 integrin with mucosal addressin cell adhesion molecule-1 (MAdCAM-1) and inhibits the migration of memory T-lymphocytes across the endothelium into inflamed gastrointestinal parenchymal tissue. Vedolizumab does not bind to or inhibit function of the α4β1 and αEβ7 integrins and does not antagonize the interaction of α4 integrins with vascular cell adhesion molecule-1 (VCAM-1).
The α4β7 integrin is expressed on the surface of a discrete subset of memory T-lymphocytes that preferentially migrate into the gastrointestinal tract. MAdCAM-1 is mainly expressed on gut endothelial cells and plays a critical role in the homing of T-lymphocytes to gut lymph tissue. The interaction of the α4β7 integrin with MAdCAM-1 has been implicated as an important contributor to the chronic inflammation that is a hallmark of ulcerative colitis and Crohn's disease.