Get your patient on Erleada (Apalutamide)
Erleada prior authorization resources
Most recent state uniform prior authorization forms
Brand Resources
Erleada patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
ERLEADA 240 mg administered orally once daily. Swallow tablets whole. ERLEADA can be taken with or without food. (2.1 , 2.3 )
Patients should also receive a gonadotropin-releasing hormone (GnRH) analog concurrently or should have had bilateral orchiectomy. (2.1 )
Recommended Dosage
The recommended dose of ERLEADA is 240 mg administered orally once daily. This could be administered as one 240 mg tablet or four 60 mg tablets. Swallow the tablet(s) whole. Do not crush or split tablet(s). ERLEADA can be taken with or without food.
Patients should also receive a gonadotropin-releasing hormone (GnRH) analog concurrently or should have had a bilateral orchiectomy.
Dosage Modification
If Grade 3 or greater adverse reactions, or other intolerable adverse reactions occur, withhold ERLEADA. Consider permanent discontinuation of ERLEADA for Grade 3 or 4 cerebrovascular and ischemic cardiovascular events [see Warnings and Precautions (5.1) ] . Permanently discontinue ERLEADA for severe ILD/pneumonitis or if no other potential causes of ILD/pneumonitis are identified, or confirmed SCARs, or for other Grade 4 skin reactions [see Warnings and Precautions (5.5 , 5.6) and Adverse Reactions (6.1) ] . For other adverse reactions, when symptoms improve to less than or equal to Grade 1 or original grade, resume ERLEADA at the same dose or a reduced dose (180 mg or 120 mg), if warranted.
Alternate Methods of Administration
Disperse Tablet(s) in Water and Administer with Orange Juice, Applesauce, or Additional Water
For patients who cannot swallow tablets whole, the recommended dose of ERLEADA tablet(s) can be dispersed in non-carbonated water and then administered with either orange juice, applesauce, or additional water as follows:
- Place the entire prescribed dose of ERLEADA tablet(s) in a cup. Do not crush or split the tablet(s).
- For one 240 mg tablet: Add about 2 teaspoons (10 mL) of non-carbonated water to make sure that the tablet is completely immersed in water.
For 60 mg tablets (prescribed dose of 240 mg, 180 mg, or 120 mg): Add about 4 teaspoons (20 mL) of non-carbonated water to make sure that the tablets are completely immersed in water. - Wait 2 minutes until the tablet(s) are broken up and spread out, then stir the mixture.
- Add 2 tablespoons (30 mL) of either orange juice, applesauce, or additional water and stir the mixture.
- Swallow the mixture immediately.
- Rinse the cup with enough water to make sure the whole dose is taken and drink it immediately.
Do not store ERLEADA that is mixed with non-carbonated water, orange juice, or applesauce for later use.
Administer Tablet(s) Through a Feeding Tube
ERLEADA tablet(s) can be administered through a feeding tube 8 French or greater as follows:
- For one 240 mg tablet: Place the tablet in the barrel of the syringe (use at least a 20 mL syringe) and draw up 10 mL of non-carbonated water into the syringe.
For 60 mg tablets (prescribed dose of 240 mg, 180 mg, or 120 mg): Place the entire prescribed dose of ERLEADA tablets in the barrel of the syringe (use at least a 50 mL syringe) and draw up 20 mL of non-carbonated water into the syringe. - Wait 10 minutes and then shake vigorously to disperse contents completely.
- Administer immediately through the feeding tube.
- Refill the syringe with non-carbonated water and administer. Repeat until no tablet residue is left in the syringe or feeding tube.

By using PrescriberAI, you agree to the AI Terms of Use.
Erleada prescribing information
INDICATIONS AND USAGE
ERLEADA is indicated for the treatment of patients with
- Metastatic castration-sensitive prostate cancer (mCSPC)
- Non-metastatic castration-resistant prostate cancer (nmCRPC)
DOSAGE AND ADMINISTRATION
ERLEADA 240 mg administered orally once daily. Swallow tablets whole. ERLEADA can be taken with or without food. (2.1 , 2.3 )
Patients should also receive a gonadotropin-releasing hormone (GnRH) analog concurrently or should have had bilateral orchiectomy. (2.1 )
Recommended Dosage
The recommended dose of ERLEADA is 240 mg administered orally once daily. This could be administered as one 240 mg tablet or four 60 mg tablets. Swallow the tablet(s) whole. Do not crush or split tablet(s). ERLEADA can be taken with or without food.
Patients should also receive a gonadotropin-releasing hormone (GnRH) analog concurrently or should have had a bilateral orchiectomy.
Dosage Modification
If Grade 3 or greater adverse reactions, or other intolerable adverse reactions occur, withhold ERLEADA. Consider permanent discontinuation of ERLEADA for Grade 3 or 4 cerebrovascular and ischemic cardiovascular events [see Warnings and Precautions (5.1) ] . Permanently discontinue ERLEADA for severe ILD/pneumonitis or if no other potential causes of ILD/pneumonitis are identified, or confirmed SCARs, or for other Grade 4 skin reactions [see Warnings and Precautions (5.5 , 5.6) and Adverse Reactions (6.1) ] . For other adverse reactions, when symptoms improve to less than or equal to Grade 1 or original grade, resume ERLEADA at the same dose or a reduced dose (180 mg or 120 mg), if warranted.
Alternate Methods of Administration
Disperse Tablet(s) in Water and Administer with Orange Juice, Applesauce, or Additional Water
For patients who cannot swallow tablets whole, the recommended dose of ERLEADA tablet(s) can be dispersed in non-carbonated water and then administered with either orange juice, applesauce, or additional water as follows:
- Place the entire prescribed dose of ERLEADA tablet(s) in a cup. Do not crush or split the tablet(s).
- For one 240 mg tablet: Add about 2 teaspoons (10 mL) of non-carbonated water to make sure that the tablet is completely immersed in water. For 60 mg tablets (prescribed dose of 240 mg, 180 mg, or 120 mg): Add about 4 teaspoons (20 mL) of non-carbonated water to make sure that the tablets are completely immersed in water.
- Wait 2 minutes until the tablet(s) are broken up and spread out, then stir the mixture.
- Add 2 tablespoons (30 mL) of either orange juice, applesauce, or additional water and stir the mixture.
- Swallow the mixture immediately.
- Rinse the cup with enough water to make sure the whole dose is taken and drink it immediately.
Do not store ERLEADA that is mixed with non-carbonated water, orange juice, or applesauce for later use.
Administer Tablet(s) Through a Feeding Tube
ERLEADA tablet(s) can be administered through a feeding tube 8 French or greater as follows:
- For one 240 mg tablet: Place the tablet in the barrel of the syringe (use at least a 20 mL syringe) and draw up 10 mL of non-carbonated water into the syringe. For 60 mg tablets (prescribed dose of 240 mg, 180 mg, or 120 mg): Place the entire prescribed dose of ERLEADA tablets in the barrel of the syringe (use at least a 50 mL syringe) and draw up 20 mL of non-carbonated water into the syringe.
- Wait 10 minutes and then shake vigorously to disperse contents completely.
- Administer immediately through the feeding tube.
- Refill the syringe with non-carbonated water and administer. Repeat until no tablet residue is left in the syringe or feeding tube.
DOSAGE FORMS AND STRENGTHS
Tablets:
- 240 mg: bluish grey to grey, oval, film-coated and debossed with "E240" on one side.
- 60 mg: slightly yellowish to greyish green, oblong, film-coated and debossed with "AR 60" on one side.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
The safety and efficacy of ERLEADA have not been established in females. Based on findings from animals and its mechanism of action, ERLEADA can cause fetal harm and loss of pregnancy when administered to a pregnant female [see Clinical Pharmacology (12.1) ] . There are no available data on ERLEADA use in pregnant women to inform a drug-associated risk. In an animal reproduction study, oral administration of apalutamide to pregnant rats during and after organogenesis resulted in fetal abnormalities and embryo-fetal lethality at maternal exposures ≥ 2 times the human clinical exposure (AUC) at the recommended dose (see Data ) .
Data
Animal Data
In a pilot embryo-fetal developmental toxicity study in rats, apalutamide caused developmental toxicity when administered at oral doses of 25, 50 or 100 mg/kg/day throughout and after the period of organogenesis (gestational days 6–20). Findings included embryo-fetal lethality (resorptions) at doses ≥50 mg/kg/day, decreased fetal anogenital distance, misshapen pituitary gland, and skeletal variations (unossified phalanges, supernumerary short thoracolumbar rib(s), and small, incomplete ossification, and/or misshapen hyoid bone) at ≥25 mg/kg/day. A dose of 100 mg/kg/day caused maternal toxicity. The doses tested in rats resulted in systemic exposures (AUC) approximately 2, 4 and 6 times, respectively, the AUC in patients.
Lactation
Risk Summary
The safety and efficacy of ERLEADA have not been established in females. There are no data on the presence of apalutamide or its metabolites in human milk, the effect on the breastfed child, or the effect on milk production.
Females and Males of Reproductive Potential
Contraception
Males
Based on the mechanism of action and findings in an animal reproduction study, advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 3 months after the last dose of ERLEADA [see Use in Specific Populations (8.1) ] .
Infertility
Males
Based on animal studies, ERLEADA may impair fertility in males of reproductive potential [see Nonclinical Toxicology (13.1) ] .
Pediatric Use
Safety and effectiveness of ERLEADA in pediatric patients have not been established.
Geriatric Use
Of the 1327 patients who received ERLEADA in clinical studies, 19% of patients were less than 65 years, 41% of patients were 65 years to 74 years, and 40% were 75 years and over.
No overall differences in effectiveness were observed between older and younger patients.
Of patients treated with ERLEADA (n=1073), Grade 3–4 adverse reactions occurred in 39% of patients younger than 65 years, 41% of patients 65–74 years, and 49% of patients 75 years or older. Falls in patients receiving ERLEADA with androgen deprivation therapy was elevated in the elderly, occurring in 8% of patients younger than 65 years, 10% of patients 65–74 years, and 19% of patients 75 years or older.
CONTRAINDICATIONS
None. (4 )
None.
WARNINGS AND PRECAUTIONS
- Cerebrovascular and ischemic cardiovascular events occurred in patients receiving ERLEADA. Monitor for signs and symptoms of cerebrovascular disorders and ischemic heart disease. Optimize management of cardiovascular risk factors. (5.1 ).
- Fractures occurred in patients receiving ERLEADA. Evaluate patients for fracture risk and treat patients with bone-targeted agents according to established guidelines. (5.2 )
- Falls occurred in patients receiving ERLEADA with increased incidence in the elderly. Evaluate patients for fall risk. (5.3 )
- Seizure occurred in 0.4% of patients receiving ERLEADA. Permanently discontinue ERLEADA in patients who develop a seizure during treatment. (5.4 )
- Severe Cutaneous Adverse Reactions (SCARs), including Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) and drug reaction with eosinophilia and systemic symptoms (DRESS), occurred in patients treated with ERLEADA. Interrupt ERLEADA if signs or symptoms of SCARs develop. Permanently discontinue if SCARs are confirmed. (5.5 )
- Interstitial Lung Disease (ILD)/pneumonitis occurred in patients treated with ERLEADA. Withhold ERLEADA for suspected ILD/pneumonitis. Permanently discontinue ERLEADA in patients with severe ILD/pneumonitis or if no other potential causes of ILD/pneumonitis are identified. (2.2 , 5.6 )
- Embryo-Fetal Toxicity: ERLEADA can cause fetal harm. Advise males with female partners of reproductive potential to use effective contraception. (5.7 , 8.1 , 8.3 )
Cerebrovascular and Ischemic Cardiovascular Events
Cerebrovascular and ischemic cardiovascular events, including events leading to death, occurred in patients receiving ERLEADA. Monitor for signs and symptoms of ischemic heart disease and cerebrovascular disorders. Optimize management of cardiovascular risk factors, such as hypertension, diabetes, or dyslipidemia. Consider discontinuation of ERLEADA for Grade 3 and 4 events.
In a randomized study (SPARTAN) of patients with nmCRPC, ischemic cardiovascular events occurred in 3.7% of patients treated with ERLEADA and 2% of patients treated with placebo. In a randomized study (TITAN) in patients with mCSPC, ischemic cardiovascular events occurred in 4.4% of patients treated with ERLEADA and 1.5% of patients treated with placebo. Across the SPARTAN and TITAN studies, 4 patients (0.3%) treated with ERLEADA, and 2 patients (0.2%) treated with placebo died from an ischemic cardiovascular event.
In the SPARTAN study, cerebrovascular events occurred in 2.5% of patients treated with ERLEADA and 1% of patients treated with placebo [see Adverse Reactions (6.1) ] . In the TITAN study, cerebrovascular events occurred in 1.9% of patients treated with ERLEADA and 2.1% of patients treated with placebo. Across the SPARTAN and TITAN studies, 3 patients (0.2%) treated with ERLEADA, and 2 patients (0.2%) treated with placebo died from a cerebrovascular event.
Patients with history of unstable angina, myocardial infarction, congestive heart failure, stroke, or transient ischemic attack within six months of randomization were excluded from the SPARTAN and TITAN studies.
Fractures
Fractures occurred in patients receiving ERLEADA. Evaluate patients for fracture risk. Monitor and manage patients at risk for fractures according to established treatment guidelines and consider use of bone-targeted agents.
In a randomized study (SPARTAN) of patients with non-metastatic castration-resistant prostate cancer, fractures occurred in 12% of patients treated with ERLEADA and in 7% of patients treated with placebo. Grade 3–4 fractures occurred in 2.7% of patients treated with ERLEADA and in 0.8% of patients treated with placebo. The median time to onset of fracture was 314 days (range: 20 to 953 days) for patients treated with ERLEADA. Routine bone density assessment and treatment of osteoporosis with bone-targeted agents were not performed in the SPARTAN study.
In a randomized study (TITAN) of patients with metastatic castration-sensitive prostate cancer, fractures occurred in 9% of patients treated with ERLEADA and in 6% of patients treated with placebo. Grade 3–4 fractures were similar in both arms at 1.5%. The median time to onset of fracture was 56 days (range: 2 to 111 days) for patients treated with ERLEADA. Routine bone density assessment and treatment of osteoporosis with bone-targeted agents were not performed in the TITAN study.
Falls
Falls occurred in patients receiving ERLEADA with increased frequency in the elderly [see Use in Specific Populations (8.5) ] . Evaluate patients for fall risk.
In a randomized study (SPARTAN), falls occurred in 16% of patients treated with ERLEADA compared to 9% of patients treated with placebo. Falls were not associated with loss of consciousness or seizure.
Seizure
Seizure occurred in patients receiving ERLEADA. Permanently discontinue ERLEADA in patients who develop a seizure during treatment. It is unknown whether anti-epileptic medications will prevent seizures with ERLEADA. Advise patients of the risk of developing a seizure while receiving ERLEADA and of engaging in any activity where sudden loss of consciousness could cause harm to themselves or others.
In two randomized studies (SPARTAN and TITAN), five patients (0.4%) treated with ERLEADA and one patient treated with placebo (0.1%) experienced a seizure. Seizure occurred from 159 to 650 days after initiation of ERLEADA. Patients with a history of seizure, predisposing factors for seizure, or receiving drugs known to decrease the seizure threshold or to induce seizure were excluded. There is no clinical experience in re-administering ERLEADA to patients who experienced a seizure.
Severe Cutaneous Adverse Reactions
Fatal and life-threatening cases of severe cutaneous adverse reactions (SCARs), including Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS), occurred in patients receiving ERLEADA [see Adverse Reactions (6.2) ] .
Monitor patients for the development of SCARs. Advise patients of the signs and symptoms of SCARs (e.g., a prodrome of fever, flu-like symptoms, mucosal lesions, progressive skin rash, or lymphadenopathy).
If a SCAR is suspected, interrupt ERLEADA until the etiology of the reaction has been determined. Consultation with a dermatologist is recommended. If a SCAR is confirmed, or for other grade 4 skin reactions, permanently discontinue ERLEADA [see Dosage and Administration (2.2) and Adverse Reactions (6.1) ] .
Interstitial Lung Disease (ILD)/Pneumonitis
Fatal and life-threatening interstitial lung disease (ILD) or pneumonitis can occur in patients treated with ERLEADA.
Post-marketing cases of ILD/pneumonitis, including fatal cases, occurred in patients treated with ERLEADA. Across clinical trials (TITAN and SPARTAN, n=1327), 0.8% of patients treated with ERLEADA experienced ILD/pneumonitis, including 0.2% who experienced Grade 3 events [see Adverse Reactions (6.1 , 6.2) ].
Monitor patients for new or worsening symptoms indicative of ILD/pneumonitis (e.g., dyspnea, cough, fever). Immediately withhold ERLEADA if ILD/pneumonitis is suspected.
Permanently discontinue ERLEADA in patients with severe ILD/pneumonitis or if no other potential causes of ILD/pneumonitis are identified [see Dosage and Administration (2.2) ] .
Embryo-Fetal Toxicity
The safety and efficacy of ERLEADA have not been established in females. Based on findings from animals and its mechanism of action, ERLEADA can cause fetal harm and loss of pregnancy when administered to a pregnant female. In an animal reproduction study, oral administration of apalutamide to pregnant rats during and after organogenesis resulted in fetal abnormalities and embryo-fetal lethality at maternal exposures ≥ 2 times the human clinical exposure (AUC) at the recommended dose. Advise males with female partners of reproductive potential to use effective contraception during treatment and for 3 months after the last dose of ERLEADA [see Use in Specific Populations (8.1 , 8.3) and Clinical Pharmacology (12.1) ] .
ADVERSE REACTIONS
The following are discussed in more detail in other sections of the labeling:
- Cerebrovascular and Ischemic Cardiovascular Events [see Warnings and Precautions (5.1) ] .
- Fractures [see Warnings and Precautions (5.2) ] .
- Falls [see Warnings and Precautions (5.3) ] .
- Seizure [see Warnings and Precautions (5.4) ] .
- Severe Cutaneous Adverse Reactions (SCARs) [see Warnings and Precautions (5.5) ] .
- Interstitial Lung Disease (ILD) [see Warnings and Precautions (5.6) ] .
Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The most common adverse reactions (≥ 10%) that occurred more frequently in the ERLEADA-treated patients (≥ 2% over placebo) from the randomized placebo-controlled clinical trials (TITAN and SPARTAN) were fatigue, arthralgia, rash, decreased appetite, fall, weight decreased, hypertension, hot flush, diarrhea, and fracture.
Metastatic Castration-sensitive Prostate Cancer (mCSPC)
TITAN, a randomized (1:1), double-blind, placebo-controlled, multi-center clinical study, enrolled patients who had mCSPC. In this study, patients received either ERLEADA at a dose of 240 mg daily or placebo. All patients in the TITAN study received a concomitant gonadotropin-releasing hormone (GnRH) analog or had prior bilateral orchiectomy. The median duration of exposure was 20 months (range: 0 to 34 months) in patients who received ERLEADA and 18 months (range: 0.1 to 34 months) in patients who received placebo.
Ten patients (1.9%) who were treated with ERLEADA died from adverse reactions. The reasons for death were ischemic cardiovascular events (n=3), acute kidney injury (n=2), cardio-respiratory arrest (n=1), sudden cardiac death (n=1), respiratory failure (n=1), cerebrovascular accident (n=1), and large intestinal ulcer perforation (n=1). ERLEADA was discontinued due to adverse reactions in 8% of patients, most commonly from rash (2.3%). Adverse reactions leading to dose interruption or reduction of ERLEADA occurred in 23% of patients; the most frequent (>1%) were rash, fatigue, and hypertension. Serious adverse reactions occurred in 20% of ERLEADA-treated patients and 20% in patients receiving placebo.
Table 1 shows adverse reactions occurring in ≥10% on the ERLEADA arm in TITAN that occurred with a ≥2% absolute increase in frequency compared to placebo. Table 2 shows laboratory abnormalities that occurred in ≥15% of patients, and more frequently (>5%) in the ERLEADA arm compared to placebo.
| ERLEADA N=524 | Placebo N=527 | |||
|---|---|---|---|---|
| System/Organ Class Adverse reaction | All Grades % | Grade 3–4 % | All Grades % | Grade 3–4 % |
| Musculoskeletal and connective tissue disorders | ||||
| Arthralgia Per the Common Terminology Criteria for Adverse Reactions (CTCAE), the highest severity for these events is Grade 3 | 17 | 0.4 | 15 | 0.9 |
| Skin and subcutaneous tissue disorders | ||||
| Rash Includes rash, rash maculo-papular, rash generalized, urticaria, rash pruritic, rash macular, conjunctivitis, erythema multiforme, rash papular, skin exfoliation, genital rash, rash erythematous, stomatitis, drug eruption, mouth ulceration, rash pustular, blister, papule, pemphigoid, skin erosion, dermatitis, and rash vesicular | 28 | 6 | 9 | 0.6 |
| Pruritus | 11 | 0.2 | 4.6 | 0.2 |
| Vascular disorders | ||||
| Hot flush | 23 | 0 | 16 | 0 |
| Hypertension | 18 | 8 | 16 | 9 |
Additional adverse reactions of interest occurring in less than 10% of patients treated with ERLEADA included diarrhea (9% versus 6% on placebo), muscle spasm (3.1% versus 1.9% on placebo), dysgeusia (3.2% versus 0.6% on placebo), hypothyroidism (3.6% versus 0.6% on placebo), and ILD/pneumonitis (1.1% versus 0.4% on placebo).
| ERLEADA N=524 | Placebo N=527 | |||
|---|---|---|---|---|
| Laboratory Abnormality | All Grades % | Grade 3–4 % | All Grades % | Grade 3–4 % |
| Hematology | ||||
| White blood cell decreased | 27 | 0.4 | 19 | 0.6 |
| Chemistry | ||||
| Hypertriglyceridemia Does not reflect fasting values | 17 | 2.5 | 12 | 2.3 |
Non-metastatic Castration-resistant Prostate Cancer (nmCRPC)
SPARTAN, a randomized (2:1), double-blind, placebo-controlled, multi-center clinical study, enrolled patients who had nmCRPC. In this study, patients received either ERLEADA at a dose of 240 mg daily or a placebo. All patients in the SPARTAN study received a concomitant gonadotropin-releasing hormone (GnRH) analog or had a bilateral orchiectomy. The median duration of exposure was 33 months (range: 0.1 to 75 months) in patients who received ERLEADA and 11 months (range: 0.1 to 37 months) in patients who received placebo.
Twenty-four patients (3%) who were treated with ERLEADA died from adverse reactions. The reasons for death with ≥ 2 patients included infection (n=7), myocardial infarction (n=3), cerebrovascular event (n=2), and unknown reason (n=3). ERLEADA was discontinued due to adverse reactions in 11% of patients, most commonly from rash (3.2%). Adverse reactions leading to dose interruption or reduction of ERLEADA occurred in 33% of patients; the most common (>1%) were rash, diarrhea, fatigue, nausea, vomiting, hypertension, and hematuria. Serious adverse reactions occurred in 25% of ERLEADA-treated patients and 23% in patients receiving placebo. The most frequent serious adverse reactions (>2%) were fracture (3.4%) in the ERLEADA arm and urinary retention (3.8%) in the placebo arm.
Table 3 shows adverse reactions occurring in ≥10% on the ERLEADA arm in SPARTAN that occurred with a ≥2% absolute increase in frequency compared to placebo. Table 4 shows laboratory abnormalities that occurred in ≥15% of patients, and more frequently (>5%) in the ERLEADA arm compared to placebo.
| ERLEADA N=803 | Placebo N=398 | |||
|---|---|---|---|---|
| System/Organ Class Adverse reaction | All Grades % | Grade 3–4 % | All Grades % | Grade 3–4 % |
| General disorders and administration site conditions | ||||
| Fatigue Includes fatigue and asthenia , Per the Common Terminology Criteria for Adverse Reactions (CTCAE), the highest severity for these events is Grade 3 | 39 | 1.4 | 28 | 0.3 |
| Musculoskeletal and connective tissue disorders | ||||
| Arthralgia | 16 | 0 | 8 | 0 |
| Skin and subcutaneous tissue disorders | ||||
| Rash Includes rash, rash maculo-papular, rash generalized, urticaria, rash pruritic, rash macular, conjunctivitis, erythema multiforme, rash papular, skin exfoliation, genital rash, rash erythematous, stomatitis, drug eruption, mouth ulceration, rash pustular, blister, papule, pemphigoid, skin erosion, dermatitis, and rash vesicular | 25 | 5.2 | 6 | 0.3 |
| Metabolism and nutrition disorders | ||||
| Decreased appetite Includes appetite disorder, decreased appetite, early satiety, and hypophagia | 12 | 0.1 | 9 | 0 |
| Peripheral edema Includes peripheral edema, generalized edema, edema, edema genital, penile edema, peripheral swelling, scrotal edema, lymphedema, swelling, and localized edema | 11 | 0 | 9 | 0 |
| Injury, poisoning and procedural complications | ||||
| Fall | 16 | 1.7 | 9 | 0.8 |
| Fracture Includes rib fracture, lumbar vertebral fracture, spinal compression fracture, spinal fracture, foot fracture, hip fracture, humerus fracture, thoracic vertebral fracture, upper limb fracture, fractured sacrum, hand fracture, pubis fracture, acetabulum fracture, ankle fracture, compression fracture, costal cartilage fracture, facial bones fracture, lower limb fracture, osteoporotic fracture, wrist fracture, avulsion fracture, fibula fracture, fractured coccyx, pelvic fracture, radius fracture, sternal fracture, stress fracture, traumatic fracture, cervical vertebral fracture, femoral neck fracture, and tibia fracture | 12 | 2.7 | 7 | 0.8 |
| Investigations | ||||
| Weight decreased | 16 | 1.1 | 6 | 0.3 |
| Vascular disorders | ||||
| Hypertension | 25 | 14 | 20 | 12 |
| Hot flush | 14 | 0 | 9 | 0 |
| Gastrointestinal disorders | ||||
| Diarrhea | 20 | 1.1 | 15 | 0.5 |
| Nausea | 18 | 0 | 16 | 0 |
Additional clinically significant adverse reactions occurring in less than 10% of patients treated with ERLEADA included hypothyroidism (8% versus 2% on placebo), pruritus (6% versus 1.5% on placebo), heart failure (2.2% versus 1% on placebo), and ILD/pneumonitis (0.6% versus 0% on placebo).
| ERLEADA N=803 | Placebo N=398 | |||
|---|---|---|---|---|
| Laboratory Abnormality | All Grades % | Grade 3–4 % | All Grades % | Grade 3–4 % |
| Hematology | ||||
| Anemia | 70 | 0.4 | 64 | 0.5 |
| Leukopenia | 47 | 0.3 | 29 | 0 |
| Lymphopenia | 41 | 1.8 | 21 | 1.6 |
| Chemistry | ||||
| Hypercholesterolemia Does not reflect fasting values | 76 | 0.1 | 46 | 0 |
| Hyperglycemia | 70 | 2 | 59 | 1.0 |
| Hypertriglyceridemia | 67 | 1.6 | 49 | 0.8 |
| Hyperkalemia | 32 | 1.9 | 22 | 0.5 |
Rash
In the combined data of two randomized, placebo-controlled clinical studies, SPARTAN and TITAN, rash associated with ERLEADA was most commonly described as macular or maculo-papular. Adverse reactions of rash were reported for 26% of patients treated with ERLEADA versus 8% of patients treated with placebo. Grade 3 rashes (defined as covering > 30% body surface area [BSA]) were reported with ERLEADA treatment (6%) versus placebo (0.5%).
The onset of rash occurred at a median of 83 days of ERLEADA treatment. Rash resolved in 78% of patients within a median of 78 days from onset of rash. Rash was commonly managed with oral antihistamines, topical corticosteroids, and 19% of patients received systemic corticosteroids. Dose reduction or dose interruption occurred in 14% and 28% of patients, respectively. Of the patients who had dose interruption, 59% experienced recurrence of rash upon reintroduction of ERLEADA.
Hypothyroidism
In the combined data of two randomized, placebo-controlled clinical studies, SPARTAN and TITAN, hypothyroidism was reported for 8% of patients treated with ERLEADA and 1.5% of patients treated with placebo based on assessments of thyroid-stimulating hormone (TSH) every 4 months. Elevated TSH occurred in 25% of patients treated with ERLEADA and 7% of patients treated with placebo. The median onset was at the first scheduled assessment. There were no Grade 3 or 4 adverse reactions. Thyroid replacement therapy was initiated in 4.9% of patients treated with ERLEADA. Thyroid replacement therapy, when clinically indicated, should be initiated or dose-adjusted [see Drug Interactions (7.2) ] .
Post-Marketing Experience
The following additional adverse reactions have been identified during post-approval use of ERLEADA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate the frequency or establish a causal relationship to drug exposure.
Respiratory, Thoracic and Mediastinal Disorders: interstitial lung disease/pneumonitis [see Warnings and Precautions (5.6) ]
Skin and Subcutaneous Tissue Disorders: Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) and drug reaction with eosinophilia and systemic symptoms (DRESS).
DRUG INTERACTIONS
Concomitant use with medications that are sensitive substrates of CYP3A4, CYP2C19, CYP2C9, UGT, P-gp, BCRP, or OATP1B1 may result in loss of activity of these medications. (7.2 )
Effect of Other Drugs on ERLEADA
Strong CYP2C8 or CYP3A4 Inhibitors
Co-administration of a strong CYP2C8 or CYP3A4 inhibitor is predicted to increase the steady-state exposure of the active moieties (sum of unbound apalutamide plus the potency-adjusted unbound N-desmethyl-apalutamide). No initial dose adjustment is necessary however, reduce the ERLEADA dose based on tolerability [see Dosage and Administration (2.2) ] . Mild or moderate inhibitors of CYP2C8 or CYP3A4 are not expected to affect the exposure of apalutamide.
Effect of ERLEADA on Other Drugs
CYP3A4, CYP2C9, CYP2C19 and UGT Substrates
ERLEADA is a strong inducer of CYP3A4 and CYP2C19, and a weak inducer of CYP2C9 in humans. Concomitant use of ERLEADA with medications that are primarily metabolized by CYP3A4, CYP2C19, or CYP2C9 can result in lower exposure to these medications. Substitution for these medications is recommended when possible or evaluate for loss of activity if medication is continued. Concomitant administration of ERLEADA with medications that are substrates of UDP-glucuronosyl transferase (UGT) can result in decreased exposure. Use caution if substrates of UGT must be co-administered with ERLEADA and evaluate for loss of activity [see Clinical Pharmacology (12.3) ] .
P-gp, BCRP or OATP1B1 Substrates
Apalutamide was shown to be a weak inducer of P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and organic anion transporting polypeptide 1B1 (OATP1B1) clinically. At steady-state, apalutamide reduced the plasma exposure to fexofenadine (a P-gp substrate) and rosuvastatin (a BCRP/OATP1B1 substrate). Concomitant use of ERLEADA with medications that are substrates of P-gp, BCRP, or OATP1B1 can result in lower exposure of these medications. Use caution if substrates of P-gp, BCRP or OATP1B1 must be co-administered with ERLEADA and evaluate for loss of activity if medication is continued [see Clinical Pharmacology (12.3) ] .
DESCRIPTION
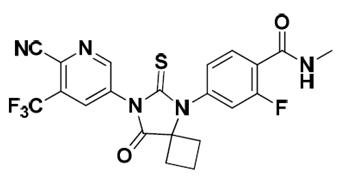
Apalutamide, the active ingredient of ERLEADA, is an androgen receptor inhibitor. Each ERLEADA tablet contains either 60 mg or 240 mg of apalutamide. The chemical name is (4-[7-(6-Cyano-5-trifluoromethylpyridin-3-yl)-8-oxo-6-thioxo-5,7-diazaspiro[3.4]oct-5-yl]-2-fluoro-N-methylbenzamide). Apalutamide is a white to slightly yellow powder. Apalutamide is practically insoluble in aqueous media over a wide range of pH values.
The molecular weight is 477.44 and molecular formula is C 21 H 15 F 4 N 5 O 2 S. The structural formula is:
ERLEADA ® (apalutamide) tablets are available in 240 mg tablets and 60 mg tablets with the following inactive ingredients:
- 240 mg film-coated tablets: colloidal anhydrous silica, croscarmellose sodium, hydroxypropyl methylcellulose-acetate succinate, silicified microcrystalline cellulose, and magnesium stearate. The coating contains glyceryl monocaprylocaprate, iron oxide black, polyvinyl alcohol, talc, titanium dioxide, and vinyl alcohol grafted copolymer.
- 60 mg film-coated tablets: colloidal anhydrous silica, croscarmellose sodium, hydroxypropyl methylcellulose-acetate succinate, magnesium stearate, microcrystalline cellulose, and silicified microcrystalline cellulose. The coating contains iron oxide black, iron oxide yellow, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide.
CLINICAL PHARMACOLOGY
Mechanism of Action
Apalutamide is an Androgen Receptor (AR) inhibitor that binds directly to the ligand-binding domain of the AR. Apalutamide inhibits AR nuclear translocation, inhibits DNA binding, and impedes AR-mediated transcription. A major metabolite, N-desmethyl apalutamide, is a less potent inhibitor of AR, and exhibited one-third the activity of apalutamide in an in vitro transcriptional reporter assay. Apalutamide administration caused decreased tumor cell proliferation and increased apoptosis leading to decreased tumor volume in mouse xenograft models of prostate cancer.
Pharmacodynamics
Apalutamide 240 mg daily in addition to ADT in patients with mCSPC (TITAN) reduced PSA to undetectable levels (<0.2 ng/mL) in 68% of patients compared to 32% of patients taking ADT alone.
Apalutamide 240 mg daily in addition to ADT in patients with nmCRPC (SPARTAN) reduced PSA to undetectable levels (<0.2 ng/mL) in 38% of patients compared to no patients (0%) taking ADT alone.
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of apalutamide have not been fully characterized.
Cardiac Electrophysiology
The effect of apalutamide 240 mg once daily on the QTc interval was assessed in an open-label, uncontrolled, multi-center, single-arm dedicated QT study in 45 patients with CRPC. The maximum mean QTcF change from baseline was 12.4 ms (2-sided 90% upper CI: 16.0 ms). An exposure-QT analysis suggested a concentration-dependent increase in QTcF for apalutamide and its active metabolite.
Pharmacokinetics
Apalutamide pharmacokinetic parameters are presented as the mean [standard deviation (SD)] unless otherwise specified. Apalutamide C max and area under the concentration curve (AUC) increased proportionally following repeated once-daily dosing of 30 to 480 mg (0.125 to 2 times the recommended dosage). Following administration of the recommended dosage, apalutamide steady-state was achieved after 4 weeks and the mean accumulation ratio was approximately 5-fold. Apalutamide C max was 6.0 mcg/mL (1.7) and AUC was 100 mcg∙h/mL (32) at steady-state. Daily fluctuations in apalutamide plasma concentrations were low, with mean peak-to-trough ratio of 1.63. An increase in apparent clearance (CL/F) was observed with repeat dosing, likely due to induction of apalutamide's own metabolism. The auto-induction effect likely reached its maximum at the recommended dosage because exposure of apalutamide across the dose range of 30 to 480 mg is dose-proportional.
The major active metabolite N-desmethyl apalutamide C max was 5.9 mcg/mL (1.0) and AUC was 124 mcg∙h/mL (23) at steady-state after the recommended dosage. N-desmethyl apalutamide was characterized by a flat concentration-time profile at steady-state with a mean peak-to-trough ratio of 1.27. Mean AUC metabolite/parent drug ratio for N-desmethyl apalutamide following repeat-dose administration was 1.3. Based on systemic exposure, relative potency, and pharmacokinetic properties, N-desmethyl apalutamide likely contributed to the clinical activity of apalutamide.
Absorption
Mean absolute oral bioavailability was approximately 100%. Median time to achieve peak plasma concentration (t max ) was 2 hours (range: 1 to 5 hours).
Oral administration of four 60 mg apalutamide tablets dispersed in applesauce resulted in no clinically relevant changes in C max and AUC when compared to administration of four intact 60 mg tablets under fasting condition.
Effect of Food
Administration of apalutamide to healthy subjects under fasting conditions and with a high-fat meal (approximately 500 to 600 fat calories, 250 carbohydrate calories, and 150 protein calories) resulted in no clinically relevant changes in C max and AUC. Median time to reach t max was delayed approximately 2 hours with food.
Distribution
The mean apparent volume of distribution at steady-state of apalutamide was approximately 276 L.
Apalutamide was 96% and N-desmethyl apalutamide was 95% bound to plasma proteins with no concentration dependency.
Elimination
The CL/F of apalutamide was 1.3 L/h after single dosing and increased to 2.0 L/h at steady-state after once-daily dosing likely due to CYP3A4 auto-induction. The mean effective half-life for apalutamide in patients was approximately 3 days at steady-state.
Metabolism
Metabolism is the main route of elimination of apalutamide. Apalutamide is primarily metabolized by CYP2C8 and CYP3A4 to form active metabolite, N-desmethyl apalutamide. The contribution of CYP2C8 and CYP3A4 in the metabolism of apalutamide is estimated to be 58% and 13% following single dose but changes to 40% and 37%, respectively at steady-state.
Apalutamide represented 45% and N-desmethyl apalutamide represented 44% of the total AUC following a single oral administration of radiolabeled apalutamide 240 mg.
Excretion
Up to 70 days following a single oral administration of radiolabeled apalutamide, 65% of the dose was recovered in urine (1.2% of dose as unchanged apalutamide and 2.7% as N-desmethyl apalutamide) and 24% was recovered in feces (1.5% of dose as unchanged apalutamide and 2% as N-desmethyl apalutamide).
Specific Populations
No clinically significant differences in the pharmacokinetics of apalutamide or N-desmethyl apalutamide were observed based on age (18–94 years), race (Black, non-Japanese Asian, Japanese), mild to moderate (eGFR 30–89 mL/min/1.73 m 2 , estimated by the modification of diet in renal disease [MDRD] equation) renal impairment, or mild (Child-Pugh A) to moderate (Child-Pugh B) hepatic impairment.
The effect of severe renal impairment or end stage renal disease (eGFR ≤29 mL/min/1.73 m 2 , MDRD) or severe hepatic impairment (Child-Pugh C) on apalutamide pharmacokinetics is unknown.
Drug Interactions
Effect of Other Drugs on ERLEADA
Strong CYP2C8 inhibitors
Apalutamide C max decreased by 21% while AUC increased by 68% following co-administration of ERLEADA as a 240 mg single dose with gemfibrozil (a strong CYP2C8 inhibitor). Gemfibrozil is predicted to increase the steady-state apalutamide C max by 32% and AUC by 44%. For the active moieties (sum of unbound apalutamide plus the potency-adjusted unbound N-desmethyl apalutamide), the predicted steady-state C max increased by 19% and AUC by 23%.
Strong CYP3A4 inhibitors
Apalutamide C max decreased by 22% while AUC was similar following co-administration of ERLEADA as a 240 mg single dose with itraconazole (a strong CYP3A4 inhibitor). Ketoconazole (a strong CYP3A4 inhibitor) is predicted to increase the single-dose apalutamide AUC by 24% but have no impact on C max . Ketoconazole is predicted to increase the steady-state apalutamide C max by 38% and AUC by 51%. For the active moieties, the predicted steady-state C max increased by 23% and AUC by 28%.
CYP3A4/CYP2C8 inducers
Rifampin (a strong CYP3A4 and moderate CYP2C8 inducer) is predicted to decrease the steady-state apalutamide C max by 25% and AUC by 34%. For the active moieties, the predicted steady-state C max decreased by 15% and AUC by 19%.
Acid lowering agents
Apalutamide is not ionizable under relevant physiological pH condition, therefore acid lowering agents (e.g., proton pump inhibitor, H 2 -receptor antagonist, antacid) are not expected to affect the solubility and bioavailability of apalutamide.
Drugs affecting transporters
In vitro , apalutamide and N-desmethyl apalutamide are substrates for P-gp but not BCRP, OATP1B1, and OATP1B3. Because apalutamide is completely absorbed after oral administration, P-gp does not limit the absorption of apalutamide and therefore, inhibition or induction of P-gp is not expected to affect the bioavailability of apalutamide.
Effect of ERLEADA on Other Drugs
CYP substrates
In vitro studies showed that apalutamide and N-desmethyl apalutamide are moderate to strong CYP3A4 and CYP2B6 inducers, are moderate inhibitors of CYP2B6 and CYP2C8, and weak inhibitors of CYP2C9, CYP2C19, and CYP3A4. Apalutamide and N-desmethyl apalutamide do not affect CYP1A2 and CYP2D6 at therapeutically relevant concentrations.
Co-administration of ERLEADA with single oral doses of sensitive CYP substrates resulted in a 92% decrease in the AUC of midazolam (a CYP3A4 substrate), 85% decrease in the AUC of omeprazole (a CYP2C19 substrate), and 46% decrease in the AUC of S-warfarin (a CYP2C9 substrate). ERLEADA did not cause clinically significant changes in exposure to a CYP2C8 substrate.
P-gp, BCRP and OATP1B1 substrates
Co-administration of ERLEADA with single oral doses of transporter substrates resulted in a 30% decrease in the AUC of fexofenadine (a P-gp substrate) and 41% decrease in the AUC of rosuvastatin (a BCRP/OATP1B1 substrate) but had no impact on C max .
UGT substrates
Apalutamide may induce UGT. Concomitant administration of ERLEADA with medications that are substrates of UGT may result in lower exposure to these medications.
OCT2, OAT1, OAT3 and MATEs substrates
In vitro , apalutamide and N-desmethyl apalutamide inhibit organic cation transporter 2 (OCT2), organic anion transporter 3 (OAT3) and multidrug and toxin extrusions (MATEs), and do not inhibit organic anion transporter 1. Apalutamide is not predicted to cause clinically significant changes in exposure to an OAT3 substrate.
GnRH analog
In mCSPC subjects receiving leuprolide acetate (a GnRH analog) co-administered with apalutamide, PK data indicated that apalutamide had no apparent effect on the steady-state exposure of leuprolide.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year carcinogenicity study in male rats, apalutamide was administered by oral gavage at doses of 5, 15 and 50 mg/kg/day. Apalutamide increased the incidence of Leydig interstitial cell adenoma in the testes at doses ≥ 5 mg/kg/day (0.2 times the human exposure based on AUC). The findings in the testes are considered to be related to the pharmacological activity of apalutamide. Rats are regarded as more sensitive than humans to developing interstitial cell tumors in the testes. Oral administration of apalutamide to male rasH2 transgenic mice for 6 months did not result in increased incidence of neoplasms at doses up to 30 mg/kg/day.
Apalutamide did not induce mutations in the bacterial reverse mutation (Ames) assay and was not genotoxic in either in vitro chromosome aberration assay or the in vivo rat bone marrow micronucleus assay or the in vivo rat Comet assay.
In repeat-dose toxicity studies in male rats (up to 26 weeks) and dogs (up to 39 weeks), atrophy of the prostate gland and seminal vesicles, aspermia/hypospermia, tubular degeneration and/or hyperplasia or hypertrophy of the interstitial cells in the reproductive system were observed at ≥ 25 mg/kg/day in rats (1.4 times the human exposure based on AUC) and ≥ 2.5 mg/kg/day in dogs (0.9 times the human exposure based on AUC).
In a fertility study in male rats, a decrease in sperm concentration and motility, increased abnormal sperm morphology, lower copulation and fertility rates (upon pairing with untreated females) along with reduced weights of the secondary sex glands and epididymis were observed following 4 weeks of dosing at ≥ 25 mg/kg/day (0.8 times the human exposure based on AUC). A reduced number of live fetuses due to increased pre- and/or post-implantation loss was observed following 4 weeks of 150 mg/kg/day administration (5.7 times the human exposure based on AUC). Effects on male rats were reversible after 8 weeks from the last apalutamide administration.
CLINICAL STUDIES
The efficacy and safety of ERLEADA was established in two randomized placebo-controlled clinical trials.
TITAN (NCT02489318): Metastatic Castration-sensitive Prostate Cancer (mCSPC)
TITAN was a randomized, double-blind, placebo-controlled, multinational, clinical trial in which 1052 patients with mCSPC were randomized (1:1) to receive either ERLEADA orally at a dose of 240 mg once daily (N=525) or placebo once daily (N=527). All patients in the TITAN trial received concomitant GnRH analog or had prior bilateral orchiectomy. Patients were stratified by Gleason score at diagnosis, prior docetaxel use, and region of the world. Patients with both high- and low-volume mCSPC were eligible for the study. High volume of disease was defined as metastases involving the viscera with 1 bone lesion or the presence of 4 or more bone lesions, at least 1 of which must be in a bony structure beyond the vertebral column and pelvic bones.
The following patient demographics and baseline disease characteristics were balanced between the treatment arms. The median age was 68 years (range 43–94) and 23% of patients were 75 years of age or older. The racial distribution was 68% Caucasian, 22% Asian, and 2% Black. Sixty-three percent (63%) of patients had high-volume disease and 37% had low-volume disease. Sixteen percent (16%) of patients had prior surgery, radiotherapy of the prostate or both. A majority of patients had a Gleason score of 8 or higher (67%). Sixty-eight percent (68%) of patients received prior treatment with an anti-androgen (bicalutamide, flutamide, or nilutamide). All patients except one in the placebo group, had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) score of 0 or 1 at study entry.
The major efficacy outcome measures of the study were overall survival (OS) and radiographic progression-free survival (rPFS). Radiographic progression-free survival was based on investigator assessment and was defined as time from randomization to radiographic disease progression or death. Radiographic disease progression was defined by identification of 2 or more new bone lesions on a bone scan with confirmation (Prostate Cancer Working Group 2 criteria) and/or progression in soft tissue disease.
A statistically significant improvement in OS and rPFS was demonstrated in patients randomized to receive ERLEADA compared with patients randomized to receive placebo. The results for OS are based upon a prespecified interim efficacy analysis. An updated OS analysis was conducted at the time of final study analysis when 405 deaths were observed. The median follow-up time was 44 months. Thirty-nine percent of patients in the placebo arm crossed over to receive ERLEADA. Efficacy results of TITAN are summarized in Table 5 and Figures 1 and 2.
| Endpoint | ERLEADA (N=525) | Placebo (N=527) |
|---|---|---|
| Primary Overall Survival Interim analysis is based on 50% of the number of events planned for the final analysis. Allocated alpha = 0.01. | ||
| Deaths (%) | 83 (16%) | 117 (22%) |
| Median, months (95% CI) NE=Not Estimable | NE (NE, NE) | NE (NE, NE) |
| Hazard Ratio (95% CI) Hazard ratio is from stratified proportional hazards model. Hazard ratio <1 favors ERLEADA. | 0.67 (0.51, 0.89) | |
| p-value p-value is from the log-rank test stratified by Gleason score at diagnosis (≤7 vs. >7), Region (NA/EU vs. Other Countries) and Prior docetaxel use (Yes vs. No). | 0.0053 | |
| Updated Overall Survival | ||
| Deaths (%) | 170 (32%) | 235 (45%) |
| Median, months (95% CI) | NE (NE, NE) | 52 (42, NE) |
| Hazard Ratio (95% CI) | 0.65 (0.53, 0.79) | |
| Radiographic Progression-free Survival | ||
| Disease progression or death (%) | 134 (26%) | 231 (44%) |
| Median, months (95% CI) | NE (NE, NE) | 22.1 (18, 33) |
| Hazard Ratio (95% CI) | 0.48 (0.39, 0.60) | |
| p-value | <0.0001 | |
Consistent improvement in rPFS was observed across the following patient subgroups: disease volume (high vs low), prior docetaxel use (yes or no), and Gleason score at diagnosis (≤7 vs. >7).
Consistent improvement in OS was observed across the following patient subgroups: disease volume (high vs low) and Gleason score at diagnosis (≤7 vs. >7).
Treatment with ERLEADA resulted in a statistically significant delay in the initiation of cytotoxic chemotherapy (HR = 0.39, 95% CI = 0.27, 0.56; p < 0.0001).
| Figure 1: Kaplan-Meier Plot of Updated Overall Survival (OS); Intent-to-treat mCSPC Population (TITAN) |
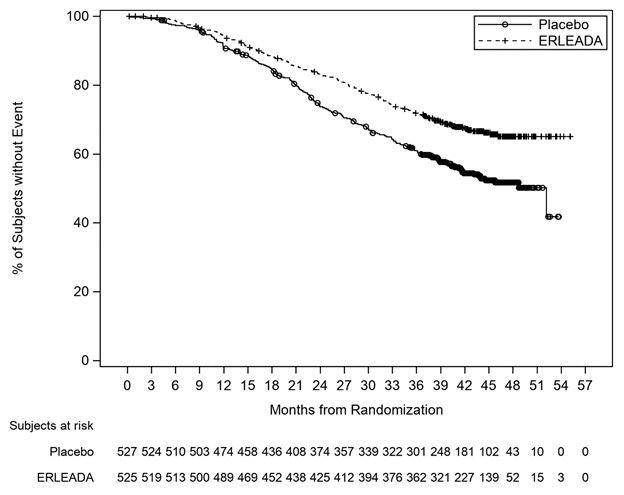 |
| Figure 2: Kaplan-Meier Plot of Radiographic Progression-Free Survival (rPFS); Intent-to-treat mCSPC Population (TITAN) |
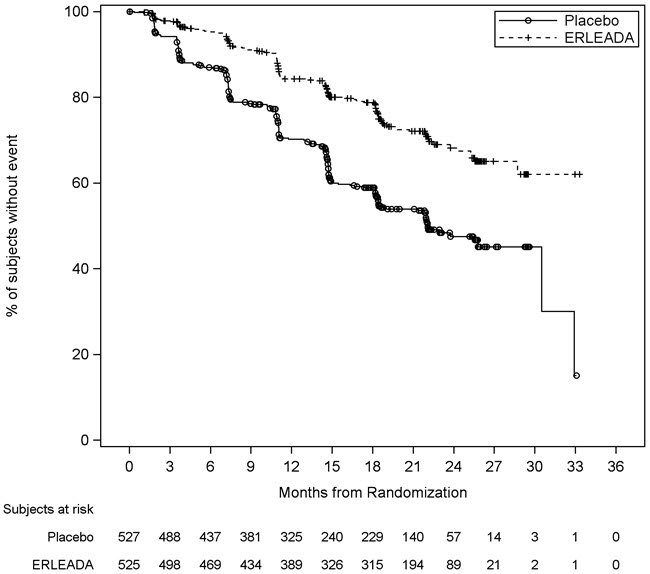 |
SPARTAN (NCT01946204): Non-metastatic, Castration-resistant Prostate Cancer (nmCRPC)
SPARTAN was a multicenter, double-blind, randomized (2:1), placebo-controlled clinical trial in which 1207 patients with nmCRPC were randomized (2:1) to receive either ERLEADA orally at a dose of 240 mg once daily (N=806) or placebo once daily (N=401). All patients in the SPARTAN trial received a concomitant GnRH analog or had a bilateral orchiectomy. Patients were stratified by Prostate Specific Antigen (PSA) Doubling Time (PSADT), the use of bone-sparing agents, and locoregional disease. Patients were required to have a PSADT ≤ 10 months and confirmation of non-metastatic disease by blinded independent central review (BICR). PSA results were blinded and were not used for treatment discontinuation. Patients randomized to either arm discontinued treatment for radiographic disease progression confirmed by BICR, locoregional-only progression, initiation of new treatment, unacceptable toxicity, or withdrawal.
The following patient demographics and baseline disease characteristics were balanced between the treatment arms. The median age was 74 years (range 48–97) and 26% of patients were 80 years of age or older. The racial distribution was 66% Caucasian, 12% Asian, and 6% Black. Seventy-seven percent (77%) of patients in both treatment arms had prior surgery or radiotherapy of the prostate. A majority of patients had a Gleason score of 7 or higher (78%). Fifteen percent (15%) of patients had <2 cm pelvic lymph nodes at study entry. Seventy-three percent (73%) of patients received prior treatment with an anti-androgen; 69% of patients received bicalutamide and 10% of patients received flutamide. All patients had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) score of 0 or 1 at study entry.
The major efficacy outcome measure of the study was metastasis-free survival (MFS), defined as the time from randomization to the time of first evidence of BICR-confirmed distant metastasis, defined as new bone or soft tissue lesions or enlarged lymph nodes above the iliac bifurcation, or death due to any cause, whichever occurred first. Additional efficacy endpoints were time to metastasis (TTM), progression-free survival (PFS) which also includes locoregional progression, time to symptomatic progression, overall survival (OS), and time to initiation of cytotoxic chemotherapy.
A statistically significant improvement in MFS and OS was demonstrated in patients randomized to receive ERLEADA compared with patients randomized to receive placebo. The major efficacy outcome (MFS) was supported by improvements in TTM and PFS. The final analysis of OS and time to initiation of cytotoxic chemotherapy was conducted 32 months after the analysis of MFS, TTM and PFS. The efficacy results from SPARTAN are summarized in Table 6 and Figures 3 and 4.
| Endpoint | ERLEADA (N=806) | Placebo (N=401) |
|---|---|---|
| Metastasis Free Survival All analyses stratified by PSA doubling time, bone-sparing agent use, and locoregional disease status. , Confirmed responses assessed by BICR. , Locoregional-only progression is observed in 2.4% of patients overall. | ||
| Number of Events (%) | 184 (23%) | 194 (48%) |
| Median, months (95% CI) NE=Not Estimable | 40.5 (NE, NE) | 16.2 (15, 18) |
| Hazard Ratio (95% CI) | 0.28 (0.23, 0.35) | |
| p-value | <0.0001 | |
| Time to Metastasis , | ||
| Number of Events (%) | 175 (22%) | 191 (48%) |
| Median, months (95% CI) | 40.5 (NE, NE) | 16.6 (15, 18) |
| Hazard Ratio (95% CI) | 0.27 (0.22, 0.34) | |
| p-value | <0.0001 | |
| Progression-Free Survival , | ||
| Number of Events (%) | 200 (25%) | 204 (51%) |
| Median, months (95% CI) | 40.5 (NE, NE) | 14.7 (14, 18) |
| Hazard Ratio (95% CI) | 0.29 (0.24, 0.36) | |
| p-value | <0.0001 | |
| Overall Survival | ||
| Number of Events (%) | 274 (34%) | 154 (38%) |
| Median, months (95% CI) | 73.9 (61, NE) | 59.9 (53, NE) |
| Hazard Ratio (95% CI) | 0.78 (0.64, 0.96) | |
| p-value | 0.0161 | |
Consistent results for MFS were observed across patient subgroups including PSADT (≤ 6 months or > 6 months), use of a prior bone-sparing agent (yes or no), and locoregional disease (N0 or N1).
Treatment with ERLEADA resulted in a statistically significant delay in the initiation of cytotoxic chemotherapy [HR = 0.63 (95% CI:0.49, 0.81), p=0.0002].
| Figure 3: Kaplan-Meier Metastasis-Free Survival (MFS) Curve in SPARTAN (nmCRPC) |
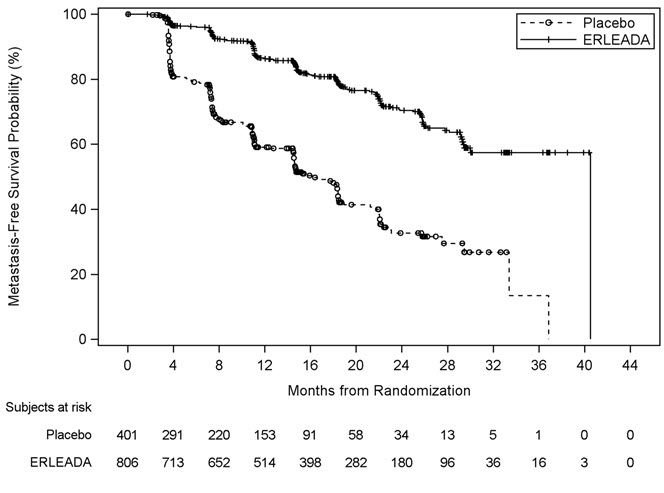 |
| Figure 4: Kaplan-Meier Overall Survival (OS) Curve in SPARTAN (nmCRPC) |
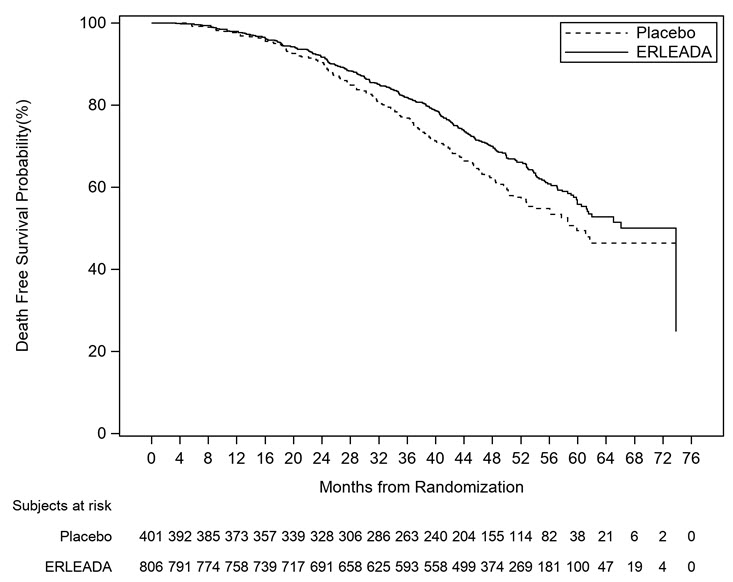 |
HOW SUPPLIED/STORAGE AND HANDLING
ERLEADA ® (apalutamide) tablets are available in the strengths and packages listed below:
- ERLEADA ® 240 mg Tablets Film coated, bluish grey to grey, oval-shaped tablets debossed with "E240" on one side. NDC Number 59676‐604‐30 - 30 tablets available in bottles with a silica gel desiccant and has a child-resistant closure
- ERLEADA ® 60 mg Tablets Film coated, slightly yellowish to greyish green, oblong-shaped tablets debossed with "AR 60" on one side. NDC Number 59676‐600‐12 - 120 tablets available in bottles with a silica gel desiccant and has a child-resistant closure
Storage and Handling
Store at 20 °C to 25 °C (68 °F to 77 °F); excursions permitted to 15 °C to 30 °C (59 °F to 86 °F) [see USP Controlled Room Temperature] .
Store in original package to protect from light and moisture. Do not discard desiccant.
| This Instructions for Use has been approved by the U.S. Food and Drug Administration. | Revised: 07/2024 | |
| INSTRUCTIONS FOR USE ERLEADA ® ( er lee'dah ) (apalutamide) tablets | ||
This Instructions for Use contains information on how to prepare and take or give a dose of ERLEADA tablets if you cannot swallow ERLEADA tablets whole or if you have a feeding tube. Read this Instructions for Use before you prepare and take or give the first dose of ERLEADA, and each time you get a refill. Ask your healthcare provider or pharmacist if you have any questions. Important information you need to know before preparing a dose of ERLEADA:
| ||
| Preparing and taking ERLEADA if you cannot swallow tablets whole: Preparing and taking ERLEADA 60 mg tablets by placing the tablets in non-carbonated water then mixing with orange juice, applesauce, or more non-carbonated water: | ||
| Step 1. | Place your entire prescribed dose of 60 mg tablets in a cup. Do not crush or split the tablets. | |
| Step 2. | Add about 4 teaspoons (20 mL) of non-carbonated water to make sure that the tablets are completely covered in water. | |
| Step 3. | Wait 2 minutes until the tablets are broken up and spread out, then stir the mixture. | |
| Step 4. | Add 2 tablespoons (30 mL) of orange juice, applesauce, or non-carbonated water to the cup and stir the mixture. | |
| Step 5. | Swallow the mixture right away. | |
| Step 6. | Rinse the cup with enough non-carbonated water to make sure that you take your full dose of ERLEADA and drink it right away. | |
| Do not store ERLEADA that is mixed with non-carbonated water, orange juice, or applesauce for later use. | ||
| Preparing and taking ERLEADA 240 mg tablet by placing the tablet in non-carbonated water then mixing with orange juice, applesauce, or more non-carbonated water: | ||
| Step 1. | Place the whole 240 mg tablet in a cup. Do not crush or split the tablet. | |
| Step 2. | Add about 2 teaspoons (10 mL) of non-carbonated water to make sure that the tablet is completely covered in water. | |
| Step 3. | Wait 2 minutes until the tablet is broken up and spread out, then stir the mixture. | |
| Step 4. | Add 2 tablespoons (30 mL) of orange juice, applesauce, or non-carbonated water to the cup and stir the mixture. | |
| Step 5. | Swallow the mixture right away. | |
| Step 6. | Rinse the cup with enough non-carbonated water to make sure that you take your full dose of ERLEADA and drink it right away. | |
| Do not store ERLEADA that is mixed with non-carbonated water, orange juice, or applesauce for later use. | ||
| Preparing and giving ERLEADA through a feeding tube: Preparing and giving ERLEADA 60 mg tablets through a feeding tube 8 French or larger: | ||
| Step 1. | Remove the plunger out of the syringe (use at least a 50 mL syringe). | |
| Step 2. | Add your entire prescribed dose of 60 mg tablets into the syringe body (barrel) and place the plunger back in the syringe. Do not crush or split the tablets. | |
| Step 3. | Withdraw 20 mL of non-carbonated water into the syringe. | |
| Step 4. | Wait 10 minutes and then shake the syringe very well (vigorously) to break the tablets apart completely. | |
| Step 5. | Attach the syringe to the feeding tube and give the mixture right away. | |
| Step 6. | Withdraw non-carbonated water into the same syringe and flush through the feeding tube. Repeat Step 6 until no pieces of tablets are left in the syringe or feeding tube. | |
| Preparing and giving ERLEADA 240 mg tablet through a feeding tube 8 French or larger: | ||
| Step 1. | Remove the plunger out of the syringe (use at least a 20 mL syringe). | |
| Step 2. | Add one 240 mg tablet into the syringe body (barrel) and place the plunger back in the syringe. Do not crush or split the tablet. | |
| Step 3. | Withdraw 10 mL of non-carbonated water into the syringe. | |
| Step 4. | Wait 10 minutes and then shake the syringe very well (vigorously) to break the tablet apart completely. | |
| Step 5. | Attach the syringe to the feeding tube and give the mixture right away. | |
| Step 6. | Withdraw non-carbonated water into the same syringe and flush through the feeding tube. Repeat Step 6 until no pieces of tablet are left in the syringe or feeding tube. | |
How should I store ERLEADA?
| ||
Mechanism of Action
Apalutamide is an Androgen Receptor (AR) inhibitor that binds directly to the ligand-binding domain of the AR. Apalutamide inhibits AR nuclear translocation, inhibits DNA binding, and impedes AR-mediated transcription. A major metabolite, N-desmethyl apalutamide, is a less potent inhibitor of AR, and exhibited one-third the activity of apalutamide in an in vitro transcriptional reporter assay. Apalutamide administration caused decreased tumor cell proliferation and increased apoptosis leading to decreased tumor volume in mouse xenograft models of prostate cancer.