Estazolam Prescribing Information
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death (see WARNINGS).
- Reserve concomitant prescribing of these drugs in patients for whom alternative treatment options are inadequate.Limit dosages and durations to the minimum required.Follow patients for signs and symptoms of respiratory depression and sedation (see WARNINGS and PRECAUTIONS).
- The use of benzodiazepines, including estazolam, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing estazolam and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (see WARNINGS).
- The continued use of benzodiazepines, including estazolam, may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Abrupt discontinuation or rapid dosage reduction of estazolam after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue estazolam or reduce the dosage (see DOSAGE AND ADMINISTRATION and WARNINGS).
Estazolam tablets, USP are indicated for the short-term management of insomnia characterized by difficulty in falling asleep, frequent nocturnal awakenings, and/or early morning awakenings. Both outpatient studies and a sleep laboratory study have shown that estazolam administered at bedtime improved sleep induction and sleep maintenance (see
Estazolam tablets have been found to be equivalent in absorption to an orally administered solution of estazolam. In healthy subjects who received up to three times the recommended dose of estazolam, peak estazolam plasma concentrations occurred within two hours after dosing (range 0.5 to 6 hours) and were proportional to the administered dose, suggesting linear pharmacokinetics over the dosage range tested.
Independent of concentration, estazolam in plasma is 93% protein bound.
Estazolam is extensively metabolized. Only two metabolites (1-oxo-estazolam and 4-hydroxy-estazolam) were detected in human plasma up to 18 hours.
The pharmacologic activity of estazolam is primarily from the parent drug. The elimination of the parent drug takes place via hepatic metabolism of estazolam to hydroxylated and other metabolites that are eliminated largely in the urine both free and conjugated. In humans, greater than 70% of a single dose of estazolam was recovered in the urine as metabolites. Less than 5% of a 2 mg dose of estazolam was excreted unchanged in the urine, with only 4% of the dose appearing in the feces. The principal urinary excretion product is an unidentified metabolite, presumed to be a metabolic product of 4-hydroxy-estazolam, accounting for at least 27% of the administered dose. 4-hydroxy-estazolam is the major metabolite in plasma, with concentrations approaching 12% of those of the parent eight hours after administration. Urinary 4-hydroxy-estazolam and 1-oxo-estazolam account for 11.9% and 4.4% of the dose respectively.
The range of estimates for the mean elimination half-life of estazolam varied from 10 to 24 hours. Radiolabel mass balance studies indicate that the main route of excretion is via the kidneys. After 5 days, 87% of the administered radioactivity was excreted in human urine. Less than 4% of the dose was excreted unchanged. Eleven metabolites were found in urine. Four metabolites were identified as 1-oxo-estazolam, 4’-hydroxy-estazolam, 4-hydroxy-estazolam, and benzophenone, as free metabolites and glucuronides. The predominant metabolite in urine (17% of the administered dose) has not been identified, but is likely to be a metabolite of 4-hydroxy-estazolam.
In a small study (N=8) using various doses in older subjects (59 to 68 years), peak estazolam concentrations were found to be similar to those observed in younger subjects with a mean elimination half-life of 18.4 hours (range 13.5 to 34.6 hours). The influence of hepatic or renal impairment on the pharmacokinetics of estazolam has not been studied.
The pharmacokinetics of estazolam have not been studied in pediatric patients.
The influence of race on the pharmacokinetics of estazolam has not been studied.
The gender-effect on the pharmacokinetics of estazolam has not been investigated.
The clearance of benzodiazepines is accelerated in smokers compared to nonsmokers, and there is evidence that this occurs with estazolam. This decrease in half-life, presumably due to enzyme induction by smoking, is consistent with other drugs with similar hepatic clearance characteristics. In all subjects and at all doses, the mean elimination half-life appeared to be independent of the dose.
The metabolism of estazolam to the major circulating metabolite 4-hydroxy-estazolam is catalyzed by CYP3A. While no
A multiple-dose study was conducted to assess the effect of fluoxetine 20 mg BID on the pharmacokinetics of estazolam 2 mg QHS after seven days. The pharmacokinetics of estazolam (Cmaxand AUC) were not affected during multiple-dose fluoxetine, suggesting no clinically significant pharmacokinetic interaction.
The results from
Because insomnia is often transient and intermittent, the prolonged administration of estazolam is generally neither necessary nor recommended. Since insomnia may be a symptom of several other disorders, the possibility that the complaint may be related to a condition for which there is a more specific treatment should be considered.
There is evidence to support the ability of estazolam to enhance the duration and quality of sleep for intervals up to 12 weeks (see
Estazolam tablets have been found to be equivalent in absorption to an orally administered solution of estazolam. In healthy subjects who received up to three times the recommended dose of estazolam, peak estazolam plasma concentrations occurred within two hours after dosing (range 0.5 to 6 hours) and were proportional to the administered dose, suggesting linear pharmacokinetics over the dosage range tested.
Independent of concentration, estazolam in plasma is 93% protein bound.
Estazolam is extensively metabolized. Only two metabolites (1-oxo-estazolam and 4-hydroxy-estazolam) were detected in human plasma up to 18 hours.
The pharmacologic activity of estazolam is primarily from the parent drug. The elimination of the parent drug takes place via hepatic metabolism of estazolam to hydroxylated and other metabolites that are eliminated largely in the urine both free and conjugated. In humans, greater than 70% of a single dose of estazolam was recovered in the urine as metabolites. Less than 5% of a 2 mg dose of estazolam was excreted unchanged in the urine, with only 4% of the dose appearing in the feces. The principal urinary excretion product is an unidentified metabolite, presumed to be a metabolic product of 4-hydroxy-estazolam, accounting for at least 27% of the administered dose. 4-hydroxy-estazolam is the major metabolite in plasma, with concentrations approaching 12% of those of the parent eight hours after administration. Urinary 4-hydroxy-estazolam and 1-oxo-estazolam account for 11.9% and 4.4% of the dose respectively.
The range of estimates for the mean elimination half-life of estazolam varied from 10 to 24 hours. Radiolabel mass balance studies indicate that the main route of excretion is via the kidneys. After 5 days, 87% of the administered radioactivity was excreted in human urine. Less than 4% of the dose was excreted unchanged. Eleven metabolites were found in urine. Four metabolites were identified as 1-oxo-estazolam, 4’-hydroxy-estazolam, 4-hydroxy-estazolam, and benzophenone, as free metabolites and glucuronides. The predominant metabolite in urine (17% of the administered dose) has not been identified, but is likely to be a metabolite of 4-hydroxy-estazolam.
In a small study (N=8) using various doses in older subjects (59 to 68 years), peak estazolam concentrations were found to be similar to those observed in younger subjects with a mean elimination half-life of 18.4 hours (range 13.5 to 34.6 hours). The influence of hepatic or renal impairment on the pharmacokinetics of estazolam has not been studied.
The pharmacokinetics of estazolam have not been studied in pediatric patients.
The influence of race on the pharmacokinetics of estazolam has not been studied.
The gender-effect on the pharmacokinetics of estazolam has not been investigated.
The clearance of benzodiazepines is accelerated in smokers compared to nonsmokers, and there is evidence that this occurs with estazolam. This decrease in half-life, presumably due to enzyme induction by smoking, is consistent with other drugs with similar hepatic clearance characteristics. In all subjects and at all doses, the mean elimination half-life appeared to be independent of the dose.
The metabolism of estazolam to the major circulating metabolite 4-hydroxy-estazolam is catalyzed by CYP3A. While no
A multiple-dose study was conducted to assess the effect of fluoxetine 20 mg BID on the pharmacokinetics of estazolam 2 mg QHS after seven days. The pharmacokinetics of estazolam (Cmaxand AUC) were not affected during multiple-dose fluoxetine, suggesting no clinically significant pharmacokinetic interaction.
The results from
The recommended initial dose for adults is 1 mg at bedtime; however, some patients may need a 2 mg dose. In healthy elderly patients, 1 mg is also the appropriate starting dose, but increases should be initiated with particular care. In small or debilitated older patients, a starting dose of 0.5 mg, while only marginally effective in the overall elderly population, should be considered.
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue estazolam or reduce the dosage. If a patient develops withdrawal reactions, consider pausing the taper or increasing the dosage to the previous tapered dosage level. Subsequently decrease the dosage more slowly (see
Estazolam is contraindicated with ketoconazole and itraconazole, since these medications significantly impair oxidative metabolism mediated by CYP3A (see
Approximately 3% of 1277 patients who received estazolam in U.S. premarketing clinical trials discontinued treatment because of an adverse clinical event. The only event commonly associated with discontinuation, accounting for 1.3% of the total, was somnolence.
(Percentage of Patients Reporting) | ||
Estazolam | Placebo | |
Body System/Adverse Event* | (N=685) | (N=433) |
| Body as a Whole | ||
Headache | 16 | 27 |
Asthenia | 11 | 8 |
Malaise | 5 | 5 |
Lower extremity pain | 3 | 2 |
Back pain | 2 | 2 |
Body pain | 2 | 2 |
Abdominal pain | 1 | 2 |
Chest pain | 1 | 1 |
| Digestive System | ||
Nausea | 4 | 5 |
Dyspepsia | 2 | 2 |
| Musculoskeletal System | ||
Stiffness | 1 | -- |
| Nervous System | ||
Somnolence | 42 | 27 |
Hypokinesia | 8 | 4 |
Nervousness | 8 | 11 |
Dizziness | 7 | 3 |
Coordination abnormal | 4 | 1 |
Hangover | 3 | 2 |
Confusion | 2 | -- |
Depression | 2 | 3 |
Dream abnormal | 2 | 2 |
Thinking abnormal | 2 | 1 |
| Respiratory System | ||
Cold symptoms | 3 | 5 |
Pharyngitis | 1 | 2 |
| Skin and Appendages | ||
Pruritus | 1 | -- |
* Events reported by at least 1% of estazolam patients. | ||
During clinical trials, some of which were not placebo-controlled, estazolam was administered to approximately 1300 patients. Untoward events associated with this exposure were recorded by clinical investigators using terminology of their own choosing. To provide a meaningful estimate of the proportion of individuals experiencing adverse events, similar types of untoward events must be grouped into a smaller number of standardized event categories. In the tabulations that follow, a standard COSTART dictionary terminology has been used to classify reported adverse events. The frequencies presented, therefore, represent the proportion of the 1277 individuals exposed to estazolam who experienced an event of the type cited on at least one occasion while receiving estazolam. All reported events are included except those already listed in the previous table, those COSTART terms too general to be informative, and those events where a drug cause was remote. Events are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent adverse events are defined as those occurring on one or more occasions in at least 1/100 patients; infrequent adverse events are those occurring in 1/100 to 1/1000 patients; rare events are those occurring in less than 1/1000 patients. It is important to emphasize that, although the events reported did occur during treatment with estazolam, they were not necessarily caused by it.
Estazolam, USP, a triazolobenzodiazepine derivative, is an oral hypnotic agent. Estazolam occurs as a fine, white, odorless powder that is soluble in alcohol and practically insoluble in water. The chemical name for estazolam is 8-chloro-6-phenyl-4
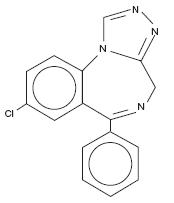
C16H11ClN4 M. W. 294.75
Each tablet, for oral administration, contains either 1 mg or 2 mg of estazolam, USP. In addition, each tablet contains the following inactive ingredients: docusate sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, sodium benzoate, sodium starch glycolate and stearic acid. The 2 mg tablets also contain FD&C Red #40 Aluminum Lake.