Estrogel prior authorization resources
Most recent state uniform prior authorization forms
Estrogel patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
2.1 Important Use Information
The timing of EstroGel initiation can affect the overall benefit-risk profile. Consider initiating EstroGel in women < 60 years old or < 10 years since menopause onset [ see Warning and Precautions (5 ), Use in Specific Populations (8.5 ) and Clinical Studies (14 ) ].
When estrogen is prescribed for a menopausal woman with a uterus, the addition of a progestogen has been shown to reduce the risk of endometrial cancer. There are possible risks associated with the use of progestogens plus estrogens that differ from those of estrogen-alone regimens. See prescribing information for progestogens indicated for the prevention of endometrial hyperplasia in non-hysterectomized menopausal women receiving estrogens [ see Warning and Precautions (5.2 , 5.3 ) ].
Generally, a woman without a uterus does not need to use a progestogen with estrogen therapy. In some cases, however, hysterectomized women with a history of endometriosis may benefit from the addition of a progestogen [ see Warning and Precautions (5.13 ) ].
2.2 Treatment of Moderate to Severe Vasomotor Symptoms due to Menopause.
EstroGel 0.06% 1.25 g per day is the single approved dose for the treatment of moderate to severe vasomotor symptoms due to menopause.
Before using the canister for the first time, it must be primed. Remove the large canister cover, and fully depress the pump 5 times. Discard the unused gel by thoroughly rinsing down the sink or placing it in the household trash. After priming, the pump is ready to use.
The recommended area of application is the arm. Apply a thin layer over the entire arm on the inside and outside from wrist to shoulder.
Treatment of Moderate to Severe Symptoms of Vulvar and Vaginal Atrophy due to Menopause.
EstroGel 0.06% 1.25 g per day is the single approved dose for the treatment of moderate to severe symptoms of vulvar and vaginal atrophy due to menopause. The lowest effective dose of EstroGel 0.06% for this indication has not been determined. When prescribing solely for the treatment of moderate to severe symptoms of vulvar and vaginal atrophy, first consider the use of topical vaginal products.
Before using the canister for the first time, it must be primed. Remove the large canister cover, and fully depress the pump 5 times. Discard the unused gel by thoroughly rinsing down the sink or placing it in the household trash. After priming, the pump is ready to use.
The recommended area of application is the arm. Apply a thin layer over the entire arm on the inside and outside from wrist to shoulder.

By using PrescriberAI, you agree to the AI Terms of Use.
Estrogel prescribing information
WARNING: ENDOMETRIAL CANCER WITH UNOPPOSED ESTROGEN IN WOMEN WITH A UTERUS
There is an increased risk of endometrial cancer in a woman with a uterus who uses unopposed estrogens. Adding a progestogen to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer.
Perform adequate diagnostic measures, including directed or random endometrial sampling when indicated, to rule out malignancy in postmenopausal women with undiagnosed, persistent, or recurring abnormal genital bleeding [see Warnings and Precautions (5.2 )].
INDICATIONS AND USAGE
EstroGel 0.06% is an estrogen indicated for:
Treatment of Moderate to Severe Vasomotor Symptoms due to Menopause.
Treatment of Moderate to Severe Symptoms of Vulvar and Vaginal Atrophy due to Menopause.
Limitation of Use When prescribing solely for the treatment of moderate to severe symptoms of vulvar and vaginal atrophy due to menopause, first consider the use of topical vaginal products.
DOSAGE AND ADMINISTRATION
2.1 Important Use Information
The timing of EstroGel initiation can affect the overall benefit-risk profile. Consider initiating EstroGel in women < 60 years old or < 10 years since menopause onset [ see Warning and Precautions (5 ), Use in Specific Populations (8.5 ) and Clinical Studies (14 ) ].
When estrogen is prescribed for a menopausal woman with a uterus, the addition of a progestogen has been shown to reduce the risk of endometrial cancer. There are possible risks associated with the use of progestogens plus estrogens that differ from those of estrogen-alone regimens. See prescribing information for progestogens indicated for the prevention of endometrial hyperplasia in non-hysterectomized menopausal women receiving estrogens [ see Warning and Precautions (5.2 , 5.3 ) ].
Generally, a woman without a uterus does not need to use a progestogen with estrogen therapy. In some cases, however, hysterectomized women with a history of endometriosis may benefit from the addition of a progestogen [ see Warning and Precautions (5.13 ) ].
2.2 Treatment of Moderate to Severe Vasomotor Symptoms due to Menopause.
EstroGel 0.06% 1.25 g per day is the single approved dose for the treatment of moderate to severe vasomotor symptoms due to menopause.
Before using the canister for the first time, it must be primed. Remove the large canister cover, and fully depress the pump 5 times. Discard the unused gel by thoroughly rinsing down the sink or placing it in the household trash. After priming, the pump is ready to use.
The recommended area of application is the arm. Apply a thin layer over the entire arm on the inside and outside from wrist to shoulder.
Treatment of Moderate to Severe Symptoms of Vulvar and Vaginal Atrophy due to Menopause.
EstroGel 0.06% 1.25 g per day is the single approved dose for the treatment of moderate to severe symptoms of vulvar and vaginal atrophy due to menopause. The lowest effective dose of EstroGel 0.06% for this indication has not been determined. When prescribing solely for the treatment of moderate to severe symptoms of vulvar and vaginal atrophy, first consider the use of topical vaginal products.
Before using the canister for the first time, it must be primed. Remove the large canister cover, and fully depress the pump 5 times. Discard the unused gel by thoroughly rinsing down the sink or placing it in the household trash. After priming, the pump is ready to use.
The recommended area of application is the arm. Apply a thin layer over the entire arm on the inside and outside from wrist to shoulder.
DOSAGE FORMS AND STRENGTHS
EstroGel 0.06% is an estradiol transdermal gel. One pump depression delivers 1.25 g of gel that contains 0.75 mg estradiol.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
EstroGel is not indicated for use in pregnancy . There are no data with the use of EstroGel in pregnant women, however, epidemiologic studies and meta-analysis have not found an increased risk of genital or non-genital birth defects (including cardiac anomalities and limb-reduction defects) following exposure to combined hormonal contraceptives (estrogens and progestins) before conception or during early pregnancy.
In the U.S. general population, the estimated background rate of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Lactation
Estrogens are present in human milk and can reduce milk production in breast-feeding women. This reduction can occur at any time but is less likely to occur once breast-feeding is well established. The development and health benefits of breastfeeding should be considered along with the mother’s clinical need for EstroGel and any potential adverse effects on the breastfed child from EstroGel or from the underlying maternal condition.
Pediatric Use
EstroGel is not indicated for use in pediatric patients. Clinical studies have not been conducted in the pediatric population.
Geriatric Use
There have not been sufficient numbers of geriatric women involved in clinical studies utilizing EstroGel to determine whether those over 65 years of age differ from younger subjects in their response to EstroGel.
The Women’s Health Initiative Studies In the WHI estrogen-alone trial (daily CE [0.625 mg]-alone versus placebo), there was a higher relative risk of stroke in women greater than 65 years of age [ see Clinical Studies (14.3 , 14.4 ) ].
The Women’s Health Initiative Memory Study In the WHIMS ancillary studies of postmenopausal women 65 to 79 years of age, there was an increased risk of probable dementia in women receiving estrogen-alone [ see Clinical Studies (14.4 , 14.5 ) ].
Since the trial was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger menopausal women [ see Clinical Studies (14.4 , 14.5 ) ]. The safety and efficacy of EstroGel for the prevention of dementia has not been established.
CONTRAINDICATIONS
EstroGel is contraindicated in women with any of the following conditions:
- Abnormal genital bleeding of unknown etiology [ see Warning and Precautions (5.2 ) ].
- Current or history of breast cancer [ see Warning and Precautions (5.2 ) ].
- Current or history of estrogen-dependent neoplasia [ see Warning and Precautions (5.2 ) ].
- Active DVT, PE, or history of these conditions [ see Warning and Precautions (5.1 ) ].
- Active arterial thromboembolic disease (for example, stroke or MI), or a history of these conditions [ see Warning and Precautions (5.1 ) ].
- Known anaphylactic reaction, angioedema, or hypersensitivity to EstroGel
- Hepatic impairment or disease [ see Warning and Precautions (5.9 ) ].
- Protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders
WARNINGS AND PRECAUTIONS
Cardiovascular Disorders
EstroGel is contraindicated in women with active DVT, PE, stroke, or a history of these conditions [ see Contraindications (4 ) ]. Immediately discontinue EstroGel if a PE, DVT, or stroke, occurs or is suspected. If feasible, discontinue EstroGel at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
The safety and efficacy of EstroGel for the prevention of cardiovascular disorders have not been established.
The Women’s Health Initiative (WHI) estrogen-alone trial reported increased risks of pulmonary embolism (PE), deep vein thrombosis (DVT), and stroke, in postmenopausal women (50 to 79 years of age, average age 63.4 years) during 7.2 years of treatment with daily oral conjugated estrogens (CE) [0.625 mg] relative to placebo. Analyses were also conducted in women aged 50-59 years, a group of women more likely to present with new onset of moderate to severe VMS compared to women of other age groups in the trial.
Only daily oral 0.625 mg CE was studied in the WHI estrogen-alone trial. Therefore, the relevance of the WHI findings regarding adverse cardiovascular events to lower CE doses, other routes of administration, or other estrogen products is not known. Without such data, it is not possible to definitively exclude these risks or determine the extent of these risks for other products [ see Clinical Studies (14.3 ) ].
Venous Thromboembolism In women aged 50-59 years, the WHI estrogen-alone trial reported a relative risk for PE of 1.53 (95% confidence interval [CI], 0.63, 3.75) for CE compared to placebo, with an absolute risk difference of 4 per 10,000 women-years (WYs; 10 versus 6). The relative risk for DVT was 1.66 (95% CI 0.75, 3.67) for CE compared to placebo, with a risk difference of 5 per 10,000 WYs (13 versus 8).
In the overall study population of women aged 50-79 years, the WHI estrogen-alone trial reported a relative risk of PE of 1.35 (95% CI 0.89, 2.05) for CE compared to placebo, with a risk difference of 4 per 10,000 WYs (14 versus 10). The relative risk for DVT was 1.48 (95% 1.06, 2.07) for CE compared to placebo, with a risk difference of 7 per 10,000 WYs (23 versus 15) [ see Clinical Studies (14.3 , 14.4 ) ].
Stroke In women aged 50-59 years, the WHI estrogen-alone trial reported a relative risk for stroke of 0.99 (95% 0.53, 1.85) for CE compared to placebo, with a risk difference of -1 per 10,000 WYs (16 versus 17). 3
In the overall study population of women aged 50-79 years, the WHI estrogen-alone trial reported a relative risk for stroke of 1.35 (95%, 1.07, 1.70) for CE compared to placebo, with a risk difference of 11 per 10,000 WYs (45 versus 34) [ see Clinical Studies (14.3 ) ]. 3
Coronary Heart Disease The WHI estrogen-alone sub-study reported no overall effect on coronary heart disease (CHD) events (defined as nonfatal MI, silent MI, or CHD death) in women receiving estrogen-alone compared to placebo 2 [ see Clinical Studies (14.4 ) ].
Subgroup analyses of women 50 to 59 years of age, who were less than 10 years since menopause, suggest a reduction (not statistically significant) of CHD events in those women receiving CE (0.625 mg)-alone compared to placebo) (8 versus 16per 10,000 women-years). 11
Malignant Neoplasms
Endometrial Cancer
In EstroGel-treated menopausal women with a uterus with persistent or recurring abnormal genital bleeding of unknown etiology, perform adequate diagnostic measures, including directed or random endometrial sampling when indicated, to assess for endometrial cancer.
An increased risk of endometrial cancer has been reported with the use of unopposed estrogen therapy in women with a uterus. The reported endometrial cancer risk among unopposed estrogen users is about 2 to 12 times greater than in non-users and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with the use of estrogens for less than 1 year. The greatest risk appears to be associated with prolonged use, with increased risks of 15- to 24-fold for 5 to 10 years or more. This risk has been shown to persist for at least 8 to 15 years after estrogen therapy is discontinued. There is no evidence that the use of natural estrogens results in a different endometrial risk profile than synthetic estrogens of equivalent estrogen dose.
Adding a progestogen to estrogen-alone therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer. There are, however, possible risks associated with the use of progestogens plus estrogens that differ from those of estrogen-alone regimens [ see Warnings and Precautions (5.3 ) ].
Breast Cancer
Surveillance measures for breast cancer, such as breast examinations and mammography, are recommended. The use of estrogen-alone therapy has been reported to result in an increase in abnormal mammograms requiring further evaluation.
In the WHI estrogen-alone trial, after an average follow-up of 7.1 years, daily CE-alone was not associated with an increased risk of invasive breast cancer. Among women 50-59 years old, the relative risk was 0.82 (95% CI, 0.50, 1.34) for CE compared to placebo, with a risk difference of -5 per 10,000 WYs (24 versus 29). In the overall study population of women aged 50-79 years (average age 63.4 years), the relative risk was 0.79 (95% CI, 0.61, 1.02), with a risk difference of -7 per 10,000 WYs (28 versus 35). [ see Clinical Studies (14.4 ) ]. However, a large meta-analysis including 24 prospective studies of postmenopausal women comparing current use of estrogen-only products with use duration of 5 to 14 years (average of 9 years) versus never use reported a relative risk for breast cancer of 1.33 (95% CI, 1.28 to 1.38). 4
Ovarian Cancer
A large meta-analysis including 17 prospective studies of postmenopausal women compared current use of estrogen-only products versus never use and reported a relative risk for ovarian cancer of 1.37 (95% CI, 1.26 to 1.50). The duration of hormone therapy use that was associated with an increased risk of ovarian cancer is unknown. 5
Risks Associated with Concomitant Use of Estrogen Plus Progestogen
If EstroGel is administered with a progestogen, there are possible risks associated with the use of progestogens plus estrogens that differ from those of estrogen-alone regimens. Refer to prescribing information for progestogens indicated for the prevention of endometrial hyperplasia in non-hysterectomized menopausal women receiving estrogens.
Gallbladder Disease
A 2- to 4-fold increase in the risk of gallbladder disease requiring surgery in postmenopausal women receiving estrogens has been reported.
Hypercalcemia
Estrogen administration may lead to severe hypercalcemia in women with breast cancer and bone metastases. Discontinue estrogens, including EstroGel if hypercalcemia occurs, and take appropriate measures to reduce the serum calcium level.
Visual Abnormalities
Retinal vascular thrombosis has been reported in women receiving estrogens. Discontinue EstroGel pending examination if there is sudden partial or complete loss of vision or a sudden onset of proptosis, diplopia, or migraine. Permanently discontinue estrogens, including EstroGel. if examination reveals papilledema or retinal vascular lesions.
5.7 Elevated Blood Pressure
In a small number of case reports, substantial increases in blood pressure have been attributed to idiosyncratic reactions to estrogens. In a large, randomized, placebo-controlled clinical trial, a generalized effect of estrogens on blood pressure was not seen.
Exacerbation of Hypertriglyceridemia
In women with pre-existing hypertriglyceridemia, estrogen therapy may be associated with elevations of plasma triglycerides leading to pancreatitis. Discontinue EstroGel if pancreatitis occurs.
5.9 Hepatic Impairment and/or Past History of Cholestatic Jaundice
Estrogens may be poorly metabolized in women with hepatic impairment. Exercise caution in any woman with a history of cholestatic jaundice associated with past estrogen use or with pregnancy. In the case of recurrence of cholestatic jaundice, discontinue EstroGel.
Exacerbation of Hypothyroidism
Estrogen administration leads to increased thyroid-binding globulin (TBG) levels. Women with normal thyroid function can compensate for the increased TBG by making more thyroid hormone, thus maintaining free T 4 and T 3 serum concentrations in the normal range. Women dependent on thyroid hormone replacement therapy who are also receiving estrogens may require increased doses of their thyroid replacement therapy. Monitor thyroid function in these women during treatment with EstroGel to maintain their free thyroid hormone levels in an acceptable range.
5.11 Fluid Retention
Estrogens may cause some degree of fluid retention. Monitor any woman with a condition(s) that might predispose her to fluid retention, such as cardiac or renal impairment., Discontinue estrogen-alone therapy, including EstroGel, with evidence of medically concerning fluid retention.
Hypocalcemia
Estrogen-induced hypocalcemia may occur in women with hypoparathyroidism. Consider whether the benefits of estrogen therapy, including EstroGel, outweigh the risks in women.
Exacerbation of Endometriosis
A few cases of malignant transformation of residual endometrial implants have been reported in women treated post-hysterectomy with estrogen-alone therapy. Consider the addition of progestogen therapy for women known to have residual endometriosis post-hysterectomy.
Hereditary Angioedema
Exogenous estrogens may exacerbate symptoms of angioedema in women with hereditary angioedema. Consider whether the benefits of estrogen therapy, including EstroGel, outweigh the risks in such women.
Exacerbation of Other Conditions
Estrogen therapy, including EstroGel, may cause an exacerbation of asthma, diabetes mellitus, epilepsy, migraine, porphyria, systemic lupus erythematosus, and hepatic hemangiomas. Consider whether the benefits of estrogen therapy outweigh the risks in women with such conditions.
Alcohol-based Products are Flammable
Avoid fire, flame, or smoking until EstroGel has dried.
Moisturizer Lotion Application
Moisturizer Lotion Application [ see Clinical Pharmacology (12.3 ) ].
Laboratory Tests
Serum follicle stimulating hormone (FSH) and estradiol levels have not been shown to be useful in the management of postmenopausal women with moderate to severe vasomotor symptoms and moderate to severe symptoms of vulvar and vaginal atrophy.
Drug-Laboratory Test Interactions
- Accelerated prothrombin time, partial thromboplastin time, and platelet aggregation time; increased platelet count; increased factors II, VII antigen, VIII antigen, VIII coagulant activity, IX, X, XII, VII-X complex, II-VII-X complex, and beta‑thromboglobulin; decreased levels of anti-factor Xa and antithrombin III, decreased antithrombin III activity; increased levels of fibrinogen and fibrinogen activity; increased plasminogen antigen and activity.
- Increased thyroid-binding globulin (TBG) levels leading to increased circulating total thyroid hormone levels, as measured by protein-bound iodine (PBI), T 4 levels (by column or by radioimmunoassay) or T 3 levels by radioimmunoassay. T 3 resin uptake is decreased, reflecting the elevated TBG. Free T 4 and T 3 concentrations are unaltered. Women on thyroid-replacement therapy may require higher doses of thyroid hormone.
- Other binding proteins may be elevated in serum (for example, corticosteroid-binding globulin [CBG], sex hormone-binding globulin [SHBG]), leading to increased total circulating corticosteroids and sex steroids, respectively. Free hormone concentrations, such as testosterone and estradiol, may be decreased. Other plasma proteins may be increased (angiotensinogen/renin substrate, alpha‑1‑antitrypsin, ceruloplasmin).
- Increased plasma high-density lipoprotein (HDL) and HDL 2 cholesterol subfraction concentrations, reduced low-density lipoprotein (LDL) cholesterol concentration, increased triglyceride levels.
- Impaired glucose tolerance.
ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling:
- Cardiovascular Disorders [see Boxed Warning , and Warnings and Precautions (5.1 )]
- Malignant Neoplasms [see Boxed Warning , and Warnings and Precautions (5.2 )]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
EstroGel was studied in 2 well-controlled, 12-week clinical trials. Incidence of adverse drug reactions ≥5 percent for 1.25 g EstroGel 0.06% and placebo is given in Table 1.
Body System/ Adverse Drug Reactions | EstroGel 0.06% 1.25 g /day (n=168) | Placebo (n=73) |
BODY AS A WHOLE | ||
Headache | 9.5 | 2.7 |
DIGESTIVE SYSTEM | ||
Flatulence | 5.4 | 4.1 |
UROGENITAL SYSTEM | ||
Breast pain | 10.7 | 8.2 |
In 2 controlled clinical trials, application site reactions were reported by 0.6 percent of patients who received 1.25 g of EstroGel. Other skin reactions, such as pruritus and rash, were also noted.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of EstroGel. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Genitourinary system
Endometrial cancer Breast Pain; tenderness; breast cancer
Cardiovascular
Deep vein thrombosis; myocardial ischemia; phlebitis
Gastrointestinal
Nausea; abdominal distension; diarrhea; stomach discomfort
Skin
Alopecia; rash; pruritus; application site: dryness, pain, discoloration, reaction, rash
Eyes
Retinal vein occlusion
Central nervous system
Headache; dizziness; insomnia; hypoesthesia; meningioma; aphasia; bradyphrenia; paresthesia
Miscellaneous
Drug ineffective; hot flush; arthralgia; night sweats; drug effect decreased; pain in extremity; fatigue; weight increased; pain; hypersensitivity; dyspnea; malignant mesenchymoma; angioedema; hepatitis acute; face edema; accidental exposure; myoclonus; gait disturbance; flushing
DRUG INTERACTIONS
In vitro and in vivo studies have shown that estrogens are metabolized partially by cytochrome P450 3A4 (CYP3A4). Therefore, inducers or inhibitors of CYP3A4 may affect estrogen drug metabolism. Inducers of CYP3A4, such as St. John’s wort ( Hypericum perforatum ) preparations, phenobarbital, carbamazepine, and rifampin, may reduce plasma concentrations of estrogens, possibly resulting in a decrease in therapeutic effects and/or changes in the uterine bleeding profile. Inhibitors of CYP3A4 such as erythromycin, clarithromycin, ketoconazole, itraconazole, ritonavir, and grapefruit juice may increase plasma concentrations of estrogen and may result in adverse reactions.
DESCRIPTION
EstroGel (estradiol gel) contains 0.06 percent estradiol in an absorptive hydroalcoholic gel base for topical application. It is a clear, colorless gel, which is odorless when dry. One pump depression of EstroGel delivers 1.25 g of gel containing 0.75 mg estradiol.
Estradiol is a white crystalline powder, chemically described as estra‑1,3,5(10)-triene-3,17β-diol. It has an empirical formula of C 18 H 24 O 2 and molecular weight of 272.39. The structural formula is:
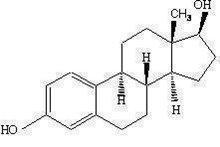
The active component of the gel is estradiol. The remaining components of the gel (purified water, alcohol, triethanolamine and carbomer 934P) are pharmacologically inactive.
CLINICAL PHARMACOLOGY
Mechanism of Action
Endogenous estrogens are largely responsible for the development and maintenance of the female reproductive system and secondary sexual characteristics. Although circulating estrogens exist in a dynamic equilibrium of metabolic interconversions, estradiol is the principal intracellular human estrogen and is substantially more potent than its metabolites, estrone and estriol, at the receptor level.
The primary source of estrogen in normally cycling adult women is the ovarian follicle, which secretes 70 to 500 mcg of estradiol daily, depending on the phase of the menstrual cycle. After menopause, most endogenous estrogen is produced by conversion of androstenedione, secreted by the adrenal cortex, to estrone in the peripheral tissues. Thus, estrone and the sulfate conjugated form, estrone sulfate, are the most abundant circulating estrogens in postmenopausal women.
Estrogens act through binding to nuclear receptors in estrogen-responsive tissues. To date, 2 estrogen receptors have been identified. These vary in proportion from tissue to tissue.
Circulating estrogens modulate the pituitary secretion of the gonadotropins, luteinizing hormone (LH) and FSH through a negative feedback mechanism. Estrogens act to reduce the elevated levels of these hormones seen in postmenopausal women.
Pharmacodynamics
Generally, a serum estrogen concentration does not predict an individual woman’s therapeutic response to EstroGel nor her risk for adverse outcomes. Likewise, exposure comparisons across different estrogen products to infer efficacy or safety for the individual woman may not be valid.
Pharmacokinetics
Absorption
Estradiol is transported across intact skin and into the systemic circulation by a passive diffusion process. The rate of diffusion across the stratum corneum is the rate- limiting factor. When EstroGel is applied to the skin, it dries in 2 to 5 minutes. EstroGel 1.25 g (containing 0.75 mg of estradiol) was administered to 24 postmenopausal women once daily on the posterior surface of 1 arm from wrist to shoulder for 14 consecutive days. Mean maximal serum concentrations of estradiol and estrone on Day 14 were 46.4 pg/mL and 64.2 pg/mL, respectively. The time-averaged serum estradiol and estrone concentrations over the 24-hour dose interval after administration of 1.25 g EstroGel on Day 14 are 28.3 pg/mL and 48.6 pg/mL, respectively. Mean concentration-time profiles for unadjusted estradiol and estrone on Day 14 are shown in Figure 1.
FIGURE 1 Mean Serum Concentration-time Profiles for Unadjusted Estradiol and Estrone After Multiple-dose Applications of 1.25 g EstroGel 0.06% for 14 Days
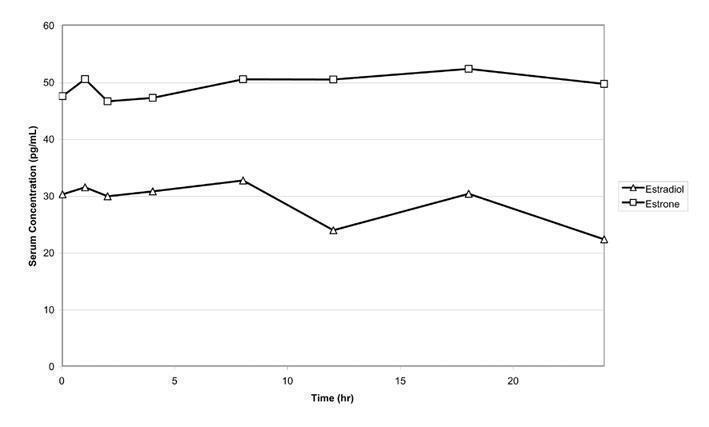
The serum concentrations of estradiol following 2.5 g EstroGel applications (1.25 g on each arm from wrist to shoulder) appeared to reach steady state after the third daily application.
Distribution
The distribution of exogenous estrogens is similar to that of endogenous estrogens. Estrogens are widely distributed in the body and are generally found in higher concentrations in the sex hormone target organs. Estrogens circulate in blood largely bound to SHBG and albumin.
Metabolism
Exogenous estrogens are metabolized in the same manner as endogenous estrogens. Circulating estrogens exist in a dynamic equilibrium of metabolic interconversions. These transformations take place mainly in the liver. Estradiol is converted reversibly to estrone, and both can be converted to estriol, which is a major urinary metabolite. Estrogens also undergo enterohepatic recirculation via sulfate and glucuronide conjugation in the liver, biliary secretion of conjugates into the intestine, and hydrolysis in the intestine followed by reabsorption. In postmenopausal women, a significant proportion of the circulating estrogens exist as sulfate conjugates, especially estrone sulfate, which serves as a circulating reservoir for the formation of more active estrogens. Although the clinical significance has not been determined, estradiol from EstroGel does not go through first-pass liver metabolism.
Excretion
Estradiol, estrone, and estriol are excreted in the urine along with glucuronide and sulfate conjugates.
The apparent terminal exponential half-life for estradiol was about 36 hours following administration of 1.25 g EstroGel.
Effect of Application Site Washing
The effect of application site washing on the serum concentrations of estradiol was determined in 24 healthy postmenopausal women who applied 1.25 g of EstroGel once daily for 14 consecutive days. Site washing 1 hour after the application resulted in a 22 percent mean decrease in average 24-hour serum concentrations of estradiol.
Potential for Estradiol Transfer
The effect of estradiol transfer was evaluated in 24 healthy postmenopausal women who topically applied 1.25 g of EstroGel once daily on the posterior surface of 1 arm from wrist to shoulder for a period of 14 consecutive days. On each day, 1 hour after gel application, a cohort of 24 non-dosed healthy postmenopausal females directly contacted the dosed cohort at the site of gel application for 15 minutes. No change in endogenous mean serum concentrations of estradiol was observed in the non-dosed cohort after direct skin-to-skin contact with subjects administered EstroGel.
Effect of Moisturizer Lotion/Sunscreen on Estradiol Absorption
The effect of sunscreen and moisturizer lotion on estradiol absorption from 0.06% estradiol topical gel was evaluated in a randomized, open-label, three-period crossover study in 42 healthy postmenopausal women. The study results showed that repeated daily application of sunscreen for 7 days at 1 hour after the administration of 0.06% estradiol topical gel decreased the mean AUC 0-24h and C max of estradiol by 16%. Repeated daily application of moisturizer lotion for 7 days at 1 hour after the administration of 0.06% estradiol topical gel increased the mean AUC 0-24h and C max of estradiol by 38% and 73%, respectively.
The effect of daily application of sunscreen/moisturizer lotion on estradiol absorption, when sunscreen/moisturizer lotion is applied before administration of 0.06% estradiol topical gel, was not studied.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term, continuous administration of natural and synthetic estrogens in certain animal species increases the frequency of carcinomas of the breast, uterus, cervix, vagina, testis, and liver.
CLINICAL STUDIES
Effects on Vasomotor Symptoms in Postmenopausal Women
In a placebo-controlled study, 145 postmenopausal women between 29 and 67 years of age (81.4 percent were White) were randomly assigned to receive 1.25 g of EstroGel (containing 0.75 mg of estradiol) or placebo gel for 12 weeks. Efficacy was assessed at 4 and 12 weeks of treatment. A statistically significant reduction in the frequency and severity of moderate to severe hot flushes was shown at Weeks 4 and 12. ( See Table 2 )
| Mean Change from Baseline in the Number and Severity of Hot Flushes per Day, ITT Population, LOCF | Number of Hot Flushes/Day (Moderate to Severe) | Severity Score/Day (Mild, Moderate, Severe) | ||
Placebo n=73 | EstroGel 0.06% 1.25 g n=72 | Placebo n=73 | EstroGel 0.06% 1.25 g n=72 | |
Baseline Mean (SD) | 11.01 (5.66) | 10.33 (3.07) | 2.30 (0.24) | 2.36 (0.29) |
Week 4 Primary timepoint. Mean (SD) Mean change from baseline (SD) Diff. vs placebo P value P values from Elteren’s nonparametric test. | 5.95 (5.17) -5.06 (4.91) | 4.43 (4.13) -5.91 (3.68) 0.85 0.019 Statistically significantly different from placebo. | 2.00 (0.63) -0.31 (0.62) | 1.73 (0.73) -0.63 (0.71) 0.32 0.005 |
Week 12 Mean (SD) Mean change from baseline (SD) Diff. vs placebo P value | 5.17 (6.52) -5.84 (4.52) | 2.79 (3.70) -7.55 (3.52) 1.71 0.043 | 1.76 (0.84) -0.54 (0.84) | 1.33 (0.97) -1.03 (0.94) 0.49 <0.001 |
Effects on Vulvar and Vaginal Atrophy in Postmenopausal Women
Results of the vaginal wall cytology showed a significant (P0.001) increase from baseline in the percent of superficial epithelial cells at Week 12 for 1.25 g EstroGel. In contrast, no significant change from baseline was observed in the placebo group.
Women’s Health Initiative Studies
The WHI enrolled approximately 27,000 predominantly healthy postmenopausal women in two substudies to assess the risks and benefits of daily oral CE (0.625 mg)-alone or in combination with MPA (2.5 mg) compared to placebo in the prevention of certain chronic diseases. The primary endpoint was the incidence of CHD (defined as nonfatal MI, silent MI, and CHD death), with invasive breast cancer as the primary adverse outcome. A “global index” included the earliest occurrence of CHD, invasive breast cancer, stroke, PE, endometrial cancer (only in the CE plus MPA substudy), colorectal cancer, hip fracture, or death due to other causes. These substudies did not evaluate the effects of CE-alone or CE plus MPA on menopausal symptoms.
WHI Estrogen-Alone Substudy The WHI estrogen-alone substudy was stopped early because an increased risk of stroke was observed, and it was deemed that no further information would be obtained regarding the risks and benefits of estrogen-alone in predetermined primary endpoints. Results of the estrogen-alone substudy, which included 10,739 women (average 63 years of age, range 50-79; 75.3 percent White, 15.1 percent Black, 6.1 percent Hispanic, 3.6 percent Other), after an average follow-up of 7.1 years are presented in Table 3.
| Relative and Absolute Risk Seen in the Estrogen-Alone Substudy of WHI a | |||
Event | Relative Risk CE vs. Placebo (95% nCI b ) | CE n = 5,310 | Placebo n = 5,429 |
Absolute Risk per 10,000 Women-Years | |||
CHD events c | 0.95 (0.78-1.16) | 54 | 57 |
Non-fatal MI c | 0.91 (0.73-1.14) | 40 | 43 |
CHD death c | 1.01 (0.71-1.43) | 16 | 16 |
All strokes c Ischemic stroke c | 1.33 (1.05-1.68) 1.55 (1.19-2.01) | 45 38 | 33 25 |
Deep vein thrombosis c,d | 1.47 (1.06-2.06) | 23 | 15 |
Pulmonary embolism c | 1.37 (0.90-2.07) | 14 | 10 |
Invasive breast cancer c | 0.80 (0.62-1.04) | 28 | 34 |
Colorectal cancer c | 1.08 (0.75-1.55) | 17 | 16 |
Hip fracture c | 0.65 (0.45-0.94) | 12 | 19 |
Vertebral fractures c,d | 0.64 (0.44-0.93) | 11 | 18 |
Lower arm/wrist fractures c,d | 0.58 (0.47-0.72) | 35 | 59 |
Total fractures c,d | 0.71 (0.64-0.80) | 144 | 197 |
Death due to other causes e,f | 1.08 (0.88-1.32) | 53 | 50 |
Overall mortality c,d | 1.04 (0.88-1.22) | 79 | 75 |
Global index g | 1.02 (0.92-1.13) | 206 | 201 |
| a Adapted from numerous WHI publications. WHI publications can be viewed at www.nhlbi.nih.gov/whi. b Nominal confidence intervals unadjusted for multiple looks and multiple comparisons. c Results are based on centrally adjudicated data for an average follow-up of 7.1 years. d Not included in “global index”. e Results are based on an average follow-up of 6.8 years. f All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease. g A subset of the events was combined in a "global index," defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, endometrial cancer, colorectal cancer, hip fracture, or death due to other causes. | |||
For those outcomes included in the WHI “global index” that reached statistical significance, the absolute excess risk per 10,000 women-years in the group treated with CE-alone was 12 more strokes, while the absolute risk reduction per 10,000 women‑years was 7 fewer hip fractures. 1 The absolute excess risk of events included in the "global index" was a non-significant 5 events per 10,000 women-years. There was no difference between the groups in terms of all-cause mortality .
No overall difference for primary CHD events (nonfatal MI, silent MI, and CHD death) and invasive breast cancer in women receiving CE-alone compared with placebo was reported in final centrally adjudicated results from the estrogen-alone substudy, after an average follow-up of 7.1 years. [See Table 3 ].
Centrally adjudicated results for stroke events from the estrogen-alone substudy, after an average follow-up of 7.1 years, reported no significant difference in the distribution of stroke subtype or severity, including fatal strokes, in women receiving CE-alone compared to placebo. Estrogen-alone therapy increased the risk of ischemic stroke, and this excess risk was present in all subgroups of women examined. 2
Timing of initiation of estrogen-alone therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen-alone substudy stratified by age showed in women 50 to 59 years of age a non-significant trend toward reduced risk for CHD [hazard ratio (HR) 0.63 (95 percent CI, 0.36-1.09)] and overall mortality [HR 0.71 (95 percent CI, 0.46-1.11)] .
Timing of initiation of estrogen plus progestin therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen plus progestin substudy stratified by age showed in women 50 to 59 years of age a non-significant trend toward reduced risk for overall mortality [HR 0.69 (95 percent CI, 0.44- 1.07)] .
Women’s Health Initiative Estrogen-Alone Trial
The WHI estrogen-alone trial enrolled predominantly healthy postmenopausal women in trial to assess the risks and benefits of daily oral CE (0.625 mg)- alone compared to placebo in the prevention of certain chronic diseases. The primary endpoint was the incidence of CHD (defined as nonfatal MI, silent MI and CHD death), with invasive breast cancer as the primary adverse outcome. A “global index” included the earliest occurrence of CHD, invasive breast cancer, stroke, PE, colorectal cancer, hip fracture, or death due to other cause. This trial did not evaluate the effects of CE-alone on menopausal symptoms.
The WHI estrogen-alone trial was stopped early because an increased risk of stroke was observed, and it was deemed that no further information would be obtained regarding the risks and benefits of estrogen-alone in predetermined primary endpoints. Centrally adjudicated results for stroke events, after an average follow- up of 7.1 years, reported estrogen-alone increased the risk for ischemic stroke compared to placebo, and this excess risk was present in all subgroups of women examined.
No overall difference for primary CHD events (nonfatal MI, silent MI and CHD death) and invasive breast cancer incidence in women receiving CE-alone compared to placebo was reported in final centrally adjudicated results from the estrogen-alone trial, after an average follow-up of 7.1 years. 3, 6,3
Results of the estrogen-alone trial, which included 10,739 women (average age of 63 years, range 50 to 79 years; 75.3% White, 15.1% Black, 6.1% Hispanic, 3.6% Other), after an average follow- up of 7.1 years are presented in Table A.
| Table A: Relative Risk and Risk Difference Observed in the WHI Estrogen-Alone Trial at an Average of 7.1 Years of Follow-up a | Event | Relative Ratio (95% CI) | Risk Difference (CE vs placebo/10,000 WYs) |
| CHD events Non-fatal MI CHD death | 0.94 (0.78-1.14) 0.97 (0.79-1.21) 1.00 (0.77-1.31) | -3 (55 vs 58) -1 (44 vs 45) 0 (29 vs 29) |
| All Strokes | 1.35 (1.07-1.70) | 11 (45 vs 34) |
| Deep vein thrombosis d | 1.48 (1.06-2.07) | 7 (23 vs 15) |
| Pulmonary embolism | 1.35 (0.89-2.05) | 4 (14 vs 10) |
| Invasive breast cancer e | 0.79 (0.61-1.02) | -7 (28 vs 35) |
| Colorectal cancer | 1.15 (0.81-1.64) | 2 (17 vs 15) |
| Hip fracture | 0.67 (0.46-0.96) | -6 (13 vs 19) |
| Vertebral fractures d | 0.64 (0.44-0.93) | -6 (12 vs 18) |
| Total fractures d | 0.72 (0.64-0.80) | -61 (153 vs 214) |
| Overall mortality c,f | 1.03 (0.88-1.21) | 3 (80 vs 77) |
| Global Index g | 1.03 (0.93-1.13) | 4 (208 vs 204) |
| a Adapted from 2013 WHI trial (CE n=5,310, placebo n=5,429). WHI publications can be viewed at www.nhlbi.nih.gov/whi. b Results are based on centrally adjudicated data. c In the WHI studies, hazard ratios were estimated using Cox proportional hazards models comparing treatment to placebo; however, they are described here as relative risks. Nominal confidence intervals unadjusted for multiple looks and multiple comparisons. d Not included in “global index.” e Includes metastatic and non-metastatic breast cancer with the exception of in situ cancer. f All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease. g A subset of the events was combined in a “global index,” defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, PE, colorectal cancer, hip fracture, or death due to other causes. | ||
Timing of the initiation of estrogen-alone therapy relative to the start of menopause may affect the overall risk benefit profile. The study results for women 50-59 years old in the WHI estrogen-alone trial are shown in Table B.
| Table B. Relative Risk and Risk Difference Observed Among Women 50-59 Years of Age in the WHI Estrogen-Alone Trial at an Average of 7.1 Years a,b | Event | Relative Ratio (95% CI) c | Risk Difference (CE vs placebo/10,000 WYs) |
| CHD events | 0.60 (0.35-1.04) | -11 (17 vs 28) | Non-fatal MI | 0.55 (0.31-1.00) | -11 (14 vs 25) | CHD death | 0.80 (0.32-2.04) | -1 (7 vs 8) |
| All Strokes | 0.99 (0.53-1.85) | -1 (16 vs 17) |
| Deep vein thrombosis d | 1.66 (0.75-3.67) | 5 (13 vs 8) |
| Pulmonary embolism | 1.53 (0.63-3.75) | 4 (10 vs 6) |
| Invasive breast cancer e | 0.82 (0.50-1.34) | -5 (24 vs 29) |
| Colorectal cancer | 0.71 (0.30-1.67) | -3 (7 vs 10) |
| Hip fracture | 5.01 (0.59-42.91) | 3 (1 vs 3) |
| Vertebral fractures d | 0.50 (0.17-1.47) | -4 (4 vs 8) |
| Total fractures d | 0.90 (0.72-1.11) | -16 (133 vs 149) |
| Overall mortality c,f | 0.70 (0.46-1.09) | -11 (29 vs 40) |
| Global Index g | 0.84 (0.66-1.07) | -19 (98 vs 117) |
| a Adapted from 2013 WHI trial (CE n=1,639; placebo=1,674). WHI publications can be viewed at www.nhlbi.nih.gov/whi b Results are based on centrally adjudicated data. c In the WHI studies, hazard ratios were estimated using Cox proportional hazards models comparing treatment to placebo; however, they are described here as relative risks. Nominal confidence intervals unadjusted for multiple looks and multiple comparisons. d Not included in “global index.” e Includes metastatic and non-metastatic breast cancer with the exception of in situ cancer. f All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease. g A subset of the events was combined in a “global index,” defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, PE, colorectal cancer, hip fracture, or death due to other causes. | ||
Women's Health Initiative Memory Study
The WHIMS estrogen-alone ancillary study of WHI enrolled 2,947 predominantly healthy hysterectomized postmenopausal women 65 to 79 years of age (45% were 65 to 69 years of age, 36% were 70 to 74 years of age, and 19% were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg)-alone on the incidence of probable dementia (primary outcome) compared to placebo. Probable dementia as defined in this study included Alzheimer’s disease (AD), vascular dementia (VaD) and mixed type (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD.
After an average follow-up of 5.2 years, the relative risk of probable dementia for CE a-lone versus placebo was 1.49 (95% CI, 0.83-2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women years. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women [ Warnings and Precautions (5.3 ) and Use in Specific Populations (8.5 ) ]. 7
HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
EstroGel is a clear, colorless, hydroalcoholic 0.06 percent estradiol gel supplied in a non aerosol, metered-dose pump. The pump consists of an LDPE inner liner encased in rigid plastic with a resealable polypropylene cap. EstroGel is available in a 50-gram (1.75 oz) size. Each individually packaged 50-gram pump contains 50 grams of gel and can deliver 30 metered 1.25-g doses.
NDC: 17139-617-40............................. (50-gram pump)
Storage and Handling
Keep out of reach of children. Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature ].
Mechanism of Action
Endogenous estrogens are largely responsible for the development and maintenance of the female reproductive system and secondary sexual characteristics. Although circulating estrogens exist in a dynamic equilibrium of metabolic interconversions, estradiol is the principal intracellular human estrogen and is substantially more potent than its metabolites, estrone and estriol, at the receptor level.
The primary source of estrogen in normally cycling adult women is the ovarian follicle, which secretes 70 to 500 mcg of estradiol daily, depending on the phase of the menstrual cycle. After menopause, most endogenous estrogen is produced by conversion of androstenedione, secreted by the adrenal cortex, to estrone in the peripheral tissues. Thus, estrone and the sulfate conjugated form, estrone sulfate, are the most abundant circulating estrogens in postmenopausal women.
Estrogens act through binding to nuclear receptors in estrogen-responsive tissues. To date, 2 estrogen receptors have been identified. These vary in proportion from tissue to tissue.
Circulating estrogens modulate the pituitary secretion of the gonadotropins, luteinizing hormone (LH) and FSH through a negative feedback mechanism. Estrogens act to reduce the elevated levels of these hormones seen in postmenopausal women.