Dosage & Administration
By using PrescriberAI, you agree to the AI Terms of Use.
Fetzima Prescribing Information
In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other antidepressant classes) that included approximately 77,000 adult patients and 4,500 pediatric patients, the incidence of suicidal thoughts and behaviors in antidepressant-treated patients aged 24 years and younger was greater than in placebo-treated patients. There was considerable variation in risk of suicidal thoughts and behaviors among drugs, but there was an increased risk identified in young patients for most drugs studied. There were differences in absolute risk of suicidal thoughts and behaviors across the different indications, with the highest incidence in patients with MDD. The drug-placebo differences in the number of cases of suicidal thoughts and behaviors per 1000 patients treated are provided in Table 1.
Age Range | Drug-Placebo Difference in Number of Patients of Suicidal Thoughts or Behaviors per 1000 Patients Treated |
Increases Compared to Placebo | |
| <18 years old | 14 additional patients |
| 18-24 years old | 5 additional patients |
Decreases Compared to Placebo | |
| 25-64 years old | 1 fewer patient |
| ≥65 years old | 6 fewer patients |
*Fetzima is not approved for use in pediatric patients.
It is unknown whether the risk of suicidal thoughts and behaviors in children, adolescents, and young adults extends to longer-term use, i.e., beyond four months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with MDD that antidepressants delay the recurrence of depression and that depression itself is a risk factor for suicidal thoughts and behaviors.
Monitor all antidepressant-treated patients for any indication for clinical worsening and emergence of suicidal thoughts and behaviors, especially during the initial few months of drug therapy, and at times of dosage changes. Counsel family members or caregivers of patients to monitor for changes in behavior and to alert the healthcare provider. Consider changing the therapeutic regimen, including possibly discontinuing FETZIMA, in patients whose depression is persistently worse, or who are experiencing emergent suicidal thoughts or behaviors.
The safety and effectiveness of FETZIMA have not been established in pediatric patients for the treatment of major depressive disorder (MDD).
The safety and efficacy of FETZIMA were evaluated in two randomized, double-blind, placebo- and active-controlled 8-week trials in pediatric patients with MDD, one in patients 7 to 17 years of age (flexible-dose study) and the other in patients 12 to 17 years of age (fixed-dose study). The primary efficacy endpoint for both studies was the change from baseline to week 8 in the Children’s Depression Rating Scale-Revised (CDRS-R) total score. The CDRS-R assesses the severity of depression and change in depressive symptoms in children and adolescents with depression. FETZIMA was not superior to placebo in either study. The most commonly observed adverse reactions in pediatric patients 7 to 17 years of age randomized to FETZIMA were similar to those observed in adults
FETZIMA was associated with an increase in blood pressure in placebo- and active-controlled trials in pediatric patients with MDD. Increases in blood pressure in pediatric patients treated with FETZIMA led to a higher proportion of pediatric patients developing new-onset and sustained hypertension when compared to adults
Antidepressants increase the risk of suicidal thoughts and behaviors in pediatric patients
In a juvenile animal study, male and female rats were treated with 10, 35, or 120 mg/kg/day of levomilnacipran by oral gavage from post-natal day 21 to 90. At 120 mg/kg/day, there was a decrease in bone mineral density in both males and females and a decrease in mean tibia length in females. These effects were not completely resolved at the end of the recovery period. There was a delay in sexual maturation in females treated with 120 mg/kg/day; however, there was no effect on fertility. The no observed adverse effect level (NOAEL) for all these findings was 35 mg/kg/day.
Warnings and Precautions (5.2 Serotonin Syndrome Serotonin-norepinephrine reuptake inhibitors (SNRIs), including FETZIMA, can precipitate serotonin syndrome, a potentially life-threatening condition. The risk is increased with concomitant use of other serotonergic drugs (including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, meperidine, methadone, tryptophan, buspirone, amphetamines, and St. John’s Wort) and with drugs that impair metabolism of serotonin, i.e., MAOIs [see Contraindications ( 4 ), Drug Interactions ( 7.1 )]. Serotonin syndrome can also occur when these drugs are used alone.Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). The concomitant use of FETZIMA with MAOIs is contraindicated. In addition, do not initiate FETZIMA in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue. No reports involved the administration of methylene blue by other routes (such as oral tablets or local tissue injection). If it is necessary to initiate treatment with a MAOI such as linezolid or intravenous methylene blue in a patient taking FETZIMA, discontinue FETZIMA before initiating treatment with the MAOI [see Dosage and Administration ( 2.5 , 2.6 ) and Contraindications ( 4 ), Drug Interactions ( 7.1 )]. Monitor all patients taking FETZIMA for the emergence of serotonin syndrome. Discontinue treatment with FETZIMA and any concomitant serotonergic agents immediately if the above events occur and initiate supportive symptomatic treatment. If concomitant use of FETZIMA with other serotonergic drugs is clinically warranted, inform patients of the increased risk for serotonin syndrome and monitor for symptoms. 5.5 Increased Risk of Bleeding Drugs that interfere with serotonin reuptake, including FETZIMA, may increase the risk of bleeding events. Concomitant use of aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), warfarin, and other anticoagulants may add to this risk. Case reports and epidemiological studies (case-control and cohort design) have demonstrated an association between use of drugs that interfere with serotonin reuptake and the occurrence of gastrointestinal bleeding. Based on data from the published observational studies, exposure to SNRIs, particularly in the month before delivery, has been associated with a less than 2-fold increase in the risk of postpartum hemorrhage [see Use in Specific Populations ( 8.1 )]. Bleeding events related to SSRIs and SNRIs have ranged from ecchymosis, hematoma, epistaxis, and petechiae to life-threatening hemorrhages.Inform patients about the increased risk of bleeding associated with the concomitant use of FETZIMA and NSAIDs, aspirin, or other drugs that affect coagulation [see Drug Interactions ( 7.1 )] . | 8/2023 |
FETZIMA® is indicated for the treatment of major depressive disorder (MDD) in adults
The efficacy of FETZIMA for the treatment of major depressive disorder (MDD) was established in three 8-week randomized, double-blind, placebo-controlled studies (at doses 40-120 mg once daily) in adult (18 - 78 years of age) outpatients who met the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) criteria for MDD. Two of the studies were fixed dose (Study 1 and Study 2) and one study
was flexible dose (Study 3).In Study 1, patients received 40 mg (n = 178), 80 mg (n = 179), or 120 mg (n = 180) of FETZIMA once daily, or placebo (n = 176). In Study 2, patients received either 40 mg (n = 188) or 80 mg (n = 188) of FETZIMA once daily, or placebo (n = 186). In the flexible-dose study (Study 3), patients received 40 to 120 mg (n = 217) of FETZIMA once daily, or placebo (n = 217) with 21%, 34%, and 44% of FETZIMA patients on 40 mg, 80 mg, and 120 mg, respectively at the end of their treatment.
In all three studies, FETZIMA demonstrated superiority over placebo in the improvement of depressive symptoms as measured by the Montgomery-Asberg Depression Rating Scale (MADRS) total score (see Table 6). FETZIMA also demonstrated superiority over placebo as measured by improvement in the Sheehan Disability Scale (SDS) functional impairment total score.
Study Number | Treatment Group | Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) | Placebo-subtracted Difference a (95% CI) |
| Study 1 (fixed dose) | FETZIMA (ER 40 mg/day)* | 36.0 (4.1) | -14.8 (1.0) | -3.2 (-5.9, -0.5) |
| FETZIMA (ER 80 mg/day)* | 36.1 (3.9) | -15.6 (1.0) | -4.0 (-6.7, -1.3) | |
| FETZIMA (ER 120 mg/day)* | 36.0 (3.9) | -16.5 (1.0) | -4.9 (-7.6, -2.1) | |
| Placebo | 35.6 (4.5) | -11.6 (1.0) | -- | |
| Study 2 (fixed-dose) | FETZIMA (ER 40 mg/day)* | 30.8 (3.4) | -14.6 (0.8) | -3.3 (-5.5, -1.1) |
| FETZIMA (ER 80 mg/day)* | 31.2 (3.5) | -14.4 (0.8) | -3.1 (-5.3, -1.0) | |
| Placebo | 31.0 (3.8) | -11.3 (0.8) | -- | |
| Study 3 (flexible-dose) | FETZIMA (ER 40 - 120 mg/day)* | 35.0 (3.6) | -15.3 (0.8) | -3.1 (-5.3, -0.9) |
| Placebo | 35.2 (3.8) | -12.2 (0.8) | -- | |
| SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval unadjusted for multiplicity. aDifference (drug minus placebo) in least-squares mean change from baseline to endpoint (Week 8). * Doses statistically significantly superior to placebo. | ||||
Post-hoc analyses of the relationships between treatment outcome and age, gender, and race did not suggest any differential responsiveness on the basis of these patient characteristics.
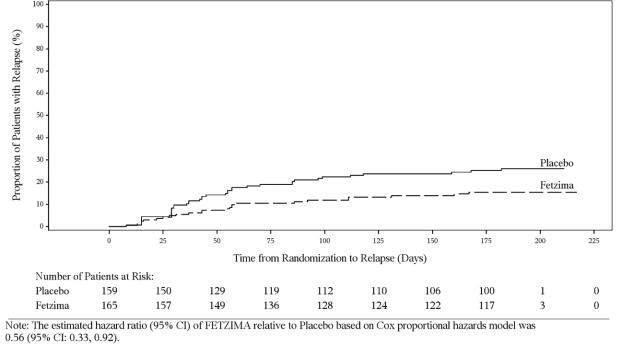
In a maintenance study (Study 4; NCT02288325), adult patients meeting DSM-5 criteria for MDD received flexibly dosed FETZIMA (40 mg, 80 mg, or 120 mg) once daily for 8 weeks. Responders in the initial 8-week treatment period were eligible to enter a 12-week, open-label, fixed-dose stabilization phase. At the end of stabilization, approximately half of the subjects were receiving 120 mg once daily. Three hundred twenty-four (324) patients who met the response criteria (MADRS total score ≤12) after 20 weeks of open-label treatment were randomized to the double-blind treatment phase with FETZIMA or placebo for 26 weeks. The primary efficacy endpoint was the time from randomization to first relapse during the double-blind phase. Relapse of depressive episode was defined as a MADRS total score ≥ 18 at two consecutive visits or insufficient therapeutic response as judged by the investigator. Patients on FETZIMA experienced a statistically significantly longer time to relapse than patients on placebo (Figure 3).

- Recommended dosage: 40 mg to 120 mg once daily with or without food ().2.1Recommended Dosage
The recommended dosage range for FETZIMA is 40 mg to 120 mg once daily, with or without food. FETZIMA should be initiated at 20 mg once daily for 2 days and then increased to 40 mg once daily. Based on clinical response and tolerability, FETZIMA may be increased in increments of 40 mg at intervals of 2 or more days. The maximum recommended dosage is 120 mg once daily.
Take FETZIMA at approximately the same time each day. Swallow FETZIMA whole; do not open, chew, or crush the capsule.
- Initial dosage is 20 mg once daily for 2 days and then increase to 40 mg once daily ().2.1Recommended Dosage
The recommended dosage range for FETZIMA is 40 mg to 120 mg once daily, with or without food. FETZIMA should be initiated at 20 mg once daily for 2 days and then increased to 40 mg once daily. Based on clinical response and tolerability, FETZIMA may be increased in increments of 40 mg at intervals of 2 or more days. The maximum recommended dosage is 120 mg once daily.
Take FETZIMA at approximately the same time each day. Swallow FETZIMA whole; do not open, chew, or crush the capsule.
- Based on clinical response and tolerability, increase dose in increments of 40 mg at intervals of 2 or more days ().2.1Recommended Dosage
The recommended dosage range for FETZIMA is 40 mg to 120 mg once daily, with or without food. FETZIMA should be initiated at 20 mg once daily for 2 days and then increased to 40 mg once daily. Based on clinical response and tolerability, FETZIMA may be increased in increments of 40 mg at intervals of 2 or more days. The maximum recommended dosage is 120 mg once daily.
Take FETZIMA at approximately the same time each day. Swallow FETZIMA whole; do not open, chew, or crush the capsule.
- The maximum recommended dosage is 120 mg once daily ().2.1Recommended Dosage
The recommended dosage range for FETZIMA is 40 mg to 120 mg once daily, with or without food. FETZIMA should be initiated at 20 mg once daily for 2 days and then increased to 40 mg once daily. Based on clinical response and tolerability, FETZIMA may be increased in increments of 40 mg at intervals of 2 or more days. The maximum recommended dosage is 120 mg once daily.
Take FETZIMA at approximately the same time each day. Swallow FETZIMA whole; do not open, chew, or crush the capsule.
- Take capsules whole; do not open, chew or crush ()2.1Recommended Dosage
The recommended dosage range for FETZIMA is 40 mg to 120 mg once daily, with or without food. FETZIMA should be initiated at 20 mg once daily for 2 days and then increased to 40 mg once daily. Based on clinical response and tolerability, FETZIMA may be increased in increments of 40 mg at intervals of 2 or more days. The maximum recommended dosage is 120 mg once daily.
Take FETZIMA at approximately the same time each day. Swallow FETZIMA whole; do not open, chew, or crush the capsule.
- Renal impairment (2.000000000000000e+003Dosage Recommendations for Patients with Renal Impairment
- End stage renal disease (ESRD): FETZIMA is not recommended;
- Severe renal impairment (creatinine clearance of 15 to 29 mL/min), the dosage should not exceed 40 mg once daily;
- Moderate renal impairment (creatinine clearance of 30 to 59 mL/min), the dosage should not exceed 80 mg once daily;
- Mild renal impairment (creatinine clearance of 60 to 89 mL/min): no dosage adjustment recommended[see Use in Specific Populations (8.7)].
):
○ Severe renal impairment: Maximum recommended dosage is 40 mg once daily.
○ Moderate renal impairment: Maximum recommended dosage is 80 mg once daily. - End stage renal disease (ESRD): FETZIMA is not recommended;
- Discontinuation: Reduce dose gradually whenever possible ()2.4DiscontinuingTreatmentwith FETZIMA
Discontinuation symptoms have been reported with discontinuation of serotonergic drugs such as FETZIMA. Gradual dose reduction is recommended, instead of abrupt discontinuation, whenever possible. Monitor patients for these symptoms when discontinuing FETZIMA. If intolerable symptoms occur following a dose decrease or upon discontinuation of treatment, consider resuming the previously prescribed dose and decreasing the dose at a more gradual rate
[see Warnings and Precautions (5.10)].
FETZIMA (levomilnacipran) is available as 20 mg, 40 mg, 80 mg, and 120 mg extended-release capsules.
Capsule Strength | Capsule Color/Shape | Capsule Markings |
| 20 mg | yellow cap white body | black "FL" on cap black "20" on body |
| 40 mg | yellow cap yellow body | black "FL" on cap black "40" on body |
| 80 mg | pink cap white body | black "FL" on cap black "80" on body |
| 120 mg | pink cap pink body | black "FL" on cap black "120" on body |
- Pregnancy:Third trimester use may increase risk for symptoms of poor adaptation (respiratory distress, temperature instability, feeding difficulty, hypotonia, tremor, irritability) in the neonate.()8.1PregnancyPregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antidepressants during pregnancy. Healthcare providers are encouraged to advise patients to register by calling the National Pregnancy Registry for Antidepressants at 1-844-405-6185 or visiting online at
https://womensmentalhealth.org/research/pregnancyregistry/antidepressants.Risk SummaryBased on data from published observational studies, exposure to SNRIs, particularly in the month before delivery, has been associated with a less than 2-fold increase in the risk of postpartum hemorrhage
[see Warnings and Precautions (5.5) and ClinicalConsiderations].The available data on FETZIMA use in pregnant women are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. There are risks associated with untreated depression in pregnancy and with exposure to SNRIs and SSRIs, including FETZIMA, during pregnancy
(seeClinical Considerations).In animal reproduction studies, levomilnacipran was not associated with malformations in rats or rabbits when given during the period of organogenesis at doses up to 8 or 16 times the maximum recommended human dose (MRHD) of 120 mg on a mg/m2basis, respectively. However, an increase in early post-natal rat pup mortality was seen at a dose equivalent to 5 times the MRHD given during pregnancy and lactation
(seeData).The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical ConsiderationsDisease-associated maternal and/or embryo/fetal riskWomen who discontinued antidepressants during pregnancy were more likely to experience a relapse of major depression than women who continued antidepressants. This finding is from a prospective, longitudinal study that followed 201 pregnant women with a history of major depressive disorder who were euthymic and taking antidepressants at the beginning of pregnancy. Consider the risk of untreated depression when discontinuing or changing treatment with antidepressant medication during pregnancy and postpartum.
Maternal Adverse ReactionsUse of FETZIMA in the month before delivery may be associated with an increased risk of postpartum hemorrhage
[see Warnings and Precautions (5.5)].Fetal/Neonatal adverse reactionsNeonates exposed to SNRIs or SSRIs, including FETZIMA, late in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding. Such complications can arise immediately upon delivery. Reported clinical findings have included respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremor, jitteriness, irritability, and constant crying. These findings are consistent with either direct toxic effect of SSRIs and SNRIs or possibly, a drug discontinuation syndrome. It should be noted that, in some cases, the clinical picture is consistent with serotonin syndrome
[see Warnings and Precautions (5.2and5.10)].DataAnimal DataNo malformations were observed when levomilnacipran was administered to pregnant rats or rabbits during the period of organogenesis at oral doses up to 100 mg/kg/day. This dose is 8 and 16 times (in rats and rabbits, respectively) the maximum recommended human dose (MRHD) of 120 mg on a mg/m2basis. Fetal body weights were reduced in rats, and skeletal ossification was delayed in both rats and rabbits at this dose; these effects were not observed in either species at doses up to 30 mg/kg/day, 2.4 times the MRHD in rats or 5 times the MRHD in rabbits on a mg/m2basis.
When levomilnacipran was administered to pregnant rats at an oral dose of 60 mg/kg/day, 5 times the MRHD, during organogenesis and throughout pregnancy and lactation, there was an increase in early postnatal pup mortality; no pup mortality was seen at 20 mg/kg/day, 1.6 times the MRHD on a mg/m2basis. Among the surviving pups, pre- and post-weaning pup weight gain was reduced up to at least 8 weeks of age; however, physical and functional development, including reproductive performance of the progeny, was not affected. The effects on body weight gain were not seen at 7 mg/kg/day, 0.6 times the MRHD on a mg/m2basis.