Fluphenazine Hydrochloride Prescribing Information
Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with neuroleptic (antipsychotic) drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of neuroleptic treatment, which patients are likely to develop the syndrome. Whether neuroleptic drug products differ in their potential to cause tardive dyskinesia is unknown.
Both the risk of developing the syndrome and the likelihood that it will become irreversible are believed to increase as the duration of treatment and the total cumulative dose of neuroleptic drugs administered to the patient increase. However, the syndrome can develop, although much less commonly, after relatively brief treatment periods at low doses.
There is no known treatment for established cases of tardive dyskinesia, although the syndrome may remit, partially or completely, if neuroleptic treatment is withdrawn. Neuroleptic treatment itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome and thereby may possibly mask the underlying disease process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown.
Given these considerations, neuroleptics should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic neuroleptic treatment should generally be reserved for patients who suffer from a chronic illness that, 1) is known to respond to neuroleptic drugs, and, 2) for whom alternative, equally effective, but potentially less harmful treatments are
If signs and symptoms of tardive dyskinesia appear in a patient on neuroleptics, drug discontinuation should be considered. However, some patients may require treatment despite the presence of the syndrome.
(For further information about the description of tardive dyskinesia and its clinical detection, please refer to the sections on
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmias).
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever and primary central nervous system (CNS) pathology.
The management of NMS should include: 1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy, 2) intensive symptomatic treatment and medical monitoring, and, 3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for uncomplicated NMS.
If a patient requires antipsychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. The patient should be carefully monitored, since recurrences of NMS have been reported.
The use of this drug may impair the mental and physical abilities required for driving a car or operating heavy machinery.
Potentiation of the effects of alcohol may occur with the use of this drug.
Since there is no adequate experience in children who have received this drug, safety and efficacy in children have not been established.
Fluphenazine hydrochloride tablets, USP may cause somnolence, postural hypotension, motor and sensory instability, which may lead to falls and, consequently, fractures or other injuries. For patients with diseases, conditions, or medications that could exacerbate these effects, complete fall risk assessments when initiating antipsychotic treatment and recurrently for patients on long-term antipsychotic therapy.
The safety for the use of this drug during pregnancy has not been established; therefore, the possible hazards should be weighed against the potential benefits when administering this drug to pregnant patients.
Non-teratogenic Effects
Neonates exposed to antipsychotic drugs, during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery. There have been reports of agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress and feeding disorder in these neonates. These complications have varied in severity; while in some cases symptoms have been self-limited, in other cases neonates have required intensive care unit support and prolonged hospitalization.
Fluphenazine hydrochloride should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Fluphenazine hydrochloride tablets are indicated in the management of manifestations of psychotic disorders.
Fluphenazine hydrochloride has not been shown effective in the management of behavioral complications in patients with mental retardation.
Depending on severity and duration of symptoms, total daily dosage for
The smallest amount that will produce the desired results must be carefully determined for each individual, since optimal dosage levels of this potent drug vary from patient to patient. In general, the oral dose has been found to be approximately 2 to 3 times the parenteral dose of fluphenazine. Treatment is best instituted with a
When symptoms are controlled, dosage can generally be reduced gradually to daily maintenance doses of 1 mg to 5 mg, often given as a single daily dose. Continued treatment is needed to achieve maximum therapeutic benefits; further adjustments in dosage may be necessary during the course of therapy to meet the patient’s requirements.
For psychotic patients who have been stabilized on a fixed daily dosage of orally administered fluphenazine hydrochloride dosage forms, conversion to the long-acting fluphenazine decanoate may be indicated (see package insert for fluphenazine decanoate for conversion information).
For geriatric patients, the suggested starting dose is 1 mg to 2.5 mg daily, adjusted according to the response of the patient.
Phenothiazines are contraindicated in patients with suspected or established subcortical brain damage, in patients receiving large doses of hypnotics, and in comatose or severely depressed states. The presence of blood dyscrasia or liver damage precludes the use of fluphenazine hydrochloride. Fluphenazine hydrochloride is contraindicated in patients who have shown hypersensitivity to fluphenazine; cross-sensitivity to phenothiazine derivatives may occur.
The side effects most frequently reported with phenothiazine compounds are extrapyramidal symptoms including pseudoparkinsonism, dystonia, dyskinesia, akathisia, oculogyric crises, opisthotonos, and hyperreflexia. Most often these extrapyramidal symptoms are reversible; however, they may be persistent (see
Extrapyramidal reactions may be alarming, and the patient should be forewarned and reassured. These reactions can usually be controlled by administration of antiparkinsonian drugs such as benztropine mesylate or intravenous caffeine and sodium benzoate injection, and by subsequent reduction in dosage.
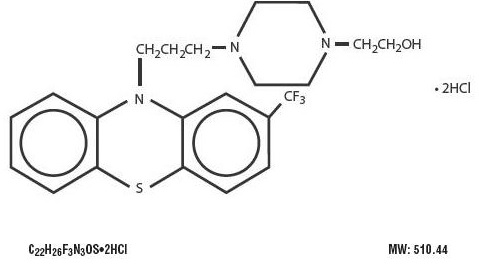
Fluphenazine hydrochloride is a trifluoromethyl phenothiazine derivative intended for the management of schizophrenia. The chemical designation is 4-[3-[2-(Trifluoromethyl) phenothiazin-10-yl] propyl]-1-piperazineethanol dihydrochloride.
The structural formula is represented below:
Fluphenazine Hydrochloride Tablets, USP, for oral administration, contain 1 mg, 2.5 mg, 5 mg, or 10 mg fluphenazine hydrochloride, USP per tablet. Each tablet also contains FD&C Blue No. 1 Aluminum Lake (2.5 mg tablet), FD&C Blue No. 2 Aluminum Lake (2.5 mg and 5 mg tablets), D&C Red No. 27 Aluminum Lake (5 mg tablet), FD&C Yellow No. 6 Aluminum Lake (10 mg tablet), lactose monohydrate, hypromellos, microcrystalline cellulose, magnesium stearate, polyethylene glycol 3350, polyvinyl alcohol, purified water, talc and titanium dioxide.