Get your patient on Flurandrenolide - Flurandrenolide lotion (Flurandrenolide)
Flurandrenolide - Flurandrenolide lotion prescribing information
INDICATIONS AND USAGE
Flurandrenolide Lotion USP, 0.05% is indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses.
DOSAGE AND ADMINISTRATION
Shake well before using. A small quantity of Flurandrenolide Lotion USP, 0.05% should be rubbed gently into the affected area 2 or 3 times daily.
Therapy should be discontinued when control is achieved. If no improvement is seen within 2 weeks, reassessment of the diagnosis may be necessary.
Flurandrenolide Lotion USP, 0.05% should not be used with occlusive dressings unless directed by a physician. Tight-fitting diapers or plastic pants may constitute occlusive dressings.
CONTRAINDICATIONS
Topical corticosteroids are contraindicated in patients with a history of hypersensitivity to any of the components of these preparations.
ADVERSE REACTIONS
The following local adverse reactions are reported infrequently with topical corticosteroids but may occur more frequently with the use of occlusive dressings. These reactions are listed in an approximate decreasing order of occurrence:
Burning
Itching
Irritation
Dryness
Folliculitis
Hypertrichosis
Acneiform eruptions
Hypopigmentation
Perioral dermatitis
Allergic contact dermatitis
The following may occur more frequently with occlusive dressings:
Maceration of the skin
Secondary infection
Skin atrophy
Striae
Miliaria
Postmarketing Adverse Reactions
The following adverse reactions have been identified during post approval use of flurandrenolide lotion. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin : skin striae, hypersensitivity, skin atrophy, contact dermatitis, and skin discoloration.
DESCRIPTION
Flurandrenolide Lotion USP, 0.05% is a potent corticosteroid intended for topical use. Flurandrenolide occurs as white to off-white, fluffy, crystalline powder and is odorless. Flurandrenolide is practically insoluble in water and in ether. One g dissolves in 72 mL of alcohol and in 10 mL of chloroform. The molecular weight of flurandrenolide is 436.52.
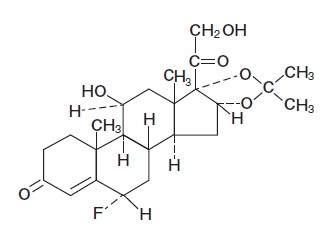
The chemical name of flurandrenolide is Pregn-4-ene-3,20-dione, 6-fluoro-11,21-dihydroxy-16,17-[(1-methylethylidene)bis (oxy)]-, (6α, 11β, 16α)-; its empirical formula is C 24 H 33 FO 6 . The structure is as follows:
Each mL of Flurandrenolide Lotion USP, 0.05% contains 0.5 mg (1.145 μmol) (0.05%) flurandrenolide in an oil-in-water emulsion base composed of glycerin, cetyl alcohol, stearic acid, glyceryl monostearate, mineral oil, polyoxyl 40 stearate, menthol, benzyl alcohol, and purified water.
CLINICAL PHARMACOLOGY
Flurandrenolide Lotion USP, 0.05% is primarily effective because of its anti-inflammatory, antipruritic, and vasoconstrictive actions.
The mechanism of the anti-inflammatory effect of topical corticosteroids is not completely understood. Various laboratory methods, including vasoconstrictor assays, are used to compare and predict potencies and/or clinical efficacies of the topical corticosteroids. There is some evidence to suggest that a recognizable correlation exists between vasoconstrictor potency and therapeutic efficacy in man. Corticosteroids with anti-inflammatory activity may stabilize cellular and lysosomal membranes. There is also the suggestion that the effect on the membranes of lysosomes prevents the release of proteolytic enzymes and, thus, plays a part in reducing inflammation.
Evaporation of water from the lotion vehicle produces a cooling effect, which is often desirable in the treatment of acutely inflamed or weeping lesions.
Pharmacokinetics -
The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings.
Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase percutaneous absorption.
Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to those of systemically administered corticosteroids. Corticosteroids are bound to plasma proteins in varying degrees. They are metabolized primarily in the liver and then excreted in the kidneys. Some of the topical corticosteroids and their metabolites are also excreted into the bile.
HOW SUPPLIED
Flurandrenolide Lotion USP, 0.05% is supplied in plastic squeeze bottles as follows:
60 mL (NDC 45802- 928 -02)
120 mL (NDC 45802- 928 -03)
Keep out of reach of children.
Storage: Avoid freezing. Keep tightly closed. Protect from light.
Store at 20° to 25°C (68° to 77°F) with excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Rx Only
Manufactured By Padagis
Yeruham, Israel
Distributed By
Padagis
Allegan, MI 49010 • www.padagis.com
Rev 02-22
2U66M RC F2