Furoscix
(Furosemide Injection 80 Mg/ 10 Ml)Dosage & Administration
FUROSCIX is intended for use in a setting where the patient can limit their activity for the duration of administration.
The On-body Infusor should not be allowed to get wet from water or any other fluids (blood or drug product). Fluid contact with the circuit board can lead to device errors and premature termination of infusion.
The On-body Infusor is intended for use in a setting where the patient can limit their activity for the duration of administration. Certain patient movements may cause interruption of device adherence to skin and premature termination of infusion.
The On-body Infusor for FUROSCIX should only be used in patients who can detect and respond to alarms to ensure a complete dose is administered.
FUROSCIX is not compatible with use in an MRI setting.
Inspect FUROSCIX prefilled cartridge prior to administration. FUROSCIX is a clear to slightly yellow solution. Do not use FUROSCIX if solution is discolored or cloudy
FUROSCIX (furosemide injection 80 mg/10 mL) is a loop diuretic which is an anthranilic acid derivative.
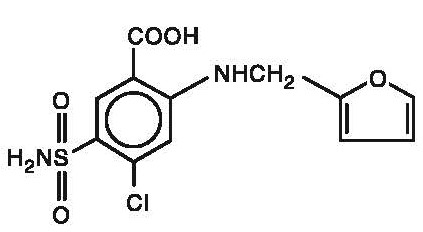
Chemically, it is 4-chloro-N-furfuryl-5-sulfamoylanthranilic acid.
Furosemide is a white to slightly yellow crystalline powder. It is sparingly soluble in alcohol, freely soluble in dilute alkali solutions and insoluble in dilute acids. The structural formula is as follows:
Molecular Formula: C12H11ClN2O5S
Molecular Weight: 330.75 g/mol
FUROSCIX is a single-dose prefilled cartridge co-packaged with a single-use, On-body Infusor. The single-dose prefilled cartridge contains 80 mg per 10 mL sterile, clear to slightly yellow, and non-pyrogenic furosemide solution. The pH of FUROSCIX, 7.4, differs from that of Furosemide Injection, USP.
Each 1 mL of FUROSCIX contains the following inactive ingredients: hydrochloric acid for pH adjustment if needed, sodium chloride (5.84 mg), sodium hydroxide for pH adjustment if needed, tris HCl (7.88 mg), and water for injection (q.s.).
FUROSCIX is administered via a wearable, single-use, electromechanical (battery powered, micro-processor controlled), On-body delivery system that is pre-programmed to deliver 80 mg of FUROSCIX over 5-hours using a bi-phasic delivery profile.
Refer to the Instructions for Use for additional information.
Load the prefilled cartridge of furosemide into the On-body Infusor and close the cartridge holder.
Peel away the adhesive liner on the On-body Infusor and apply onto a clean, dry area of the abdomen between the top of the beltline and the bottom of the ribcage that is not tender, bruised, red or indurated. The distance from the top of the beltline to the bottom of the ribcage should be at least 2 ½ inches.
Start the injection by firmly pressing and releasing the blue start button.
Do not remove until the injection is complete (signaled by the solid green status light, beeping sound, and the white plunger rod filling the cartridge window).
Rotate the site of each subcutaneous administration.
By using PrescriberAI, you agree to the AI Terms of Use.
Furoscix Prescribing Information
FUROSCIX is indicated for the treatment of edema in adult patients with chronic heart failure or chronic kidney disease (CKD), including the nephrotic syndrome.
FUROSCIX is intended for use in a setting where the patient can limit their activity for the duration of administration.
The On-body Infusor should not be allowed to get wet from water or any other fluids (blood or drug product). Fluid contact with the circuit board can lead to device errors and premature termination of infusion.
The On-body Infusor is intended for use in a setting where the patient can limit their activity for the duration of administration. Certain patient movements may cause interruption of device adherence to skin and premature termination of infusion.
The On-body Infusor for FUROSCIX should only be used in patients who can detect and respond to alarms to ensure a complete dose is administered.
FUROSCIX is not compatible with use in an MRI setting.
Inspect FUROSCIX prefilled cartridge prior to administration. FUROSCIX is a clear to slightly yellow solution. Do not use FUROSCIX if solution is discolored or cloudy
FUROSCIX (furosemide injection 80 mg/10 mL) is a loop diuretic which is an anthranilic acid derivative.
Chemically, it is 4-chloro-N-furfuryl-5-sulfamoylanthranilic acid.
Furosemide is a white to slightly yellow crystalline powder. It is sparingly soluble in alcohol, freely soluble in dilute alkali solutions and insoluble in dilute acids. The structural formula is as follows:
Molecular Formula: C12H11ClN2O5S
Molecular Weight: 330.75 g/mol
FUROSCIX is a single-dose prefilled cartridge co-packaged with a single-use, On-body Infusor. The single-dose prefilled cartridge contains 80 mg per 10 mL sterile, clear to slightly yellow, and non-pyrogenic furosemide solution. The pH of FUROSCIX, 7.4, differs from that of Furosemide Injection, USP.
Each 1 mL of FUROSCIX contains the following inactive ingredients: hydrochloric acid for pH adjustment if needed, sodium chloride (5.84 mg), sodium hydroxide for pH adjustment if needed, tris HCl (7.88 mg), and water for injection (q.s.).
FUROSCIX is administered via a wearable, single-use, electromechanical (battery powered, micro-processor controlled), On-body delivery system that is pre-programmed to deliver 80 mg of FUROSCIX over 5-hours using a bi-phasic delivery profile.

Refer to the Instructions for Use for additional information.
Load the prefilled cartridge of furosemide into the On-body Infusor and close the cartridge holder.
Peel away the adhesive liner on the On-body Infusor and apply onto a clean, dry area of the abdomen between the top of the beltline and the bottom of the ribcage that is not tender, bruised, red or indurated. The distance from the top of the beltline to the bottom of the ribcage should be at least 2 ½ inches.
Start the injection by firmly pressing and releasing the blue start button.
Do not remove until the injection is complete (signaled by the solid green status light, beeping sound, and the white plunger rod filling the cartridge window).
Rotate the site of each subcutaneous administration.
Injection: 80 mg/10 mL (8 mg/mL) as a clear to slightly yellow solution in a single-dose prefilled cartridge for use only with co-packaged single-use, On-body Infusor.
In patients with hepatic cirrhosis and ascites, sudden alterations of fluid and electrolyte balance may precipitate hepatic encephalopathy and coma. Treat such patients in a setting where clinical status and electrolyte balance can be carefully monitored.
- FUROSCIX is contraindicated in patients with anuria.
- FUROSCIX is contraindicated in patients with a history of hypersensitivity to furosemide, any component of the FUROSCIX formulation, or medical adhesives.
The On-body Infusor should not be allowed to get wet from water or any other fluids (blood or drug product). Fluid contact with the circuit board can lead to device errors and premature termination of infusion.
The On-body Infusor is intended for use in a setting where the patient can limit their activity for the duration of administration. Certain patient movements may cause interruption of device adherence to skin and premature termination of infusion.
The On-body Infusor for FUROSCIX should only be used in patients who can detect and respond to alarms to ensure a complete dose is administered.