Giapreza
(Angiotensin Ii)Dosage & Administration
Dilute GIAPREZA in 0.9% sodium chloride prior to use. See Full Prescribing Information for instructions on preparation and administration of injection. Diluted solution may be stored at room temperature or under refrigeration and should be discarded after 24 hours. GIAPREZA must be administered as an intravenous infusion. (
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Discard vial and any unused portion of the drug product after use.
Diluted solution may be stored at room temperature (20°C to 25°C [68°F to 77°F]) or under refrigeration (2°C to 8°C [36°F to 46°F]). Discard prepared solution after 24 hours at room temperature or under refrigeration.
By using PrescriberAI, you agree to the AI Terms of Use.
Giapreza Prescribing Information
Dosage and Administration, Preparation (Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. GIAPREZA must be administered as an intravenous infusion. GIAPREZA must be diluted in 0.9% sodium chloride prior to use. Dilute the appropriate amount of GIAPREZA in a normal saline (0.9% sodium chloride) infusion bag to achieve the desired final concentration of 5,000 ng/mL or 10,000 ng/mL. Discard vial and any unused portion of the drug product after use. Diluted solution may be stored at room temperature (20°C to 25°C [68°F to 77°F]) or under refrigeration (2°C to 8°C [36°F to 46°F]). Discard prepared solution after 24 hours at room temperature or under refrigeration. | 12/2021 |
GIAPREZA increases blood pressure in adults with septic or other distributive shock
The Angiotensin II for the Treatment of High-Output Shock (ATHOS-3) trial was a double-blind study in which 321 adults with septic or other distributive shock who remained hypotensive despite fluid and vasopressor therapy were randomized 1:1 and treated with either GIAPREZA or placebo, both in addition to background vasopressor therapy. Doses of GIAPREZA or placebo were titrated to a target MAP of ≥ 75 mmHg during the first 3 hours of treatment while doses of other vasopressors were maintained. From Hour 3 to Hour 48, GIAPREZA or placebo were titrated to maintain MAP between 65 and 70 mmHg while reducing doses of other vasopressors. The primary endpoint was the percentage of subjects who achieved either a MAP ≥ 75 mmHg or a ≥ 10 mmHg increase in MAP without an increase in baseline vasopressor therapy at 3 hours.
91% of subjects had septic shock; the remaining subjects had other forms of distributive shock such as neurogenic shock. At the time of study drug administration, 97% of subjects were receiving norepinephrine, 67% vasopressin, 15% phenylephrine, 13% epinephrine, and 2% dopamine. 83% of subjects had received two or more vasopressors and 47% three or more vasopressors prior to study drug administration. 61% of subjects were male, 80% were White, 10% were Black, and 10% were other races. The median age of subjects was 64 years (range: 22-89 years). Patients requiring high doses of steroids, patients with a history of asthma or bronchospasm, and patients with Raynaud's syndrome were not included.
The primary endpoint was achieved by 70% of patients randomized to GIAPREZA compared to 23% of placebo patients; p < 0.0001 (a treatment effect of 47%). Figure 1 shows the results in all patients and in selected subgroups.
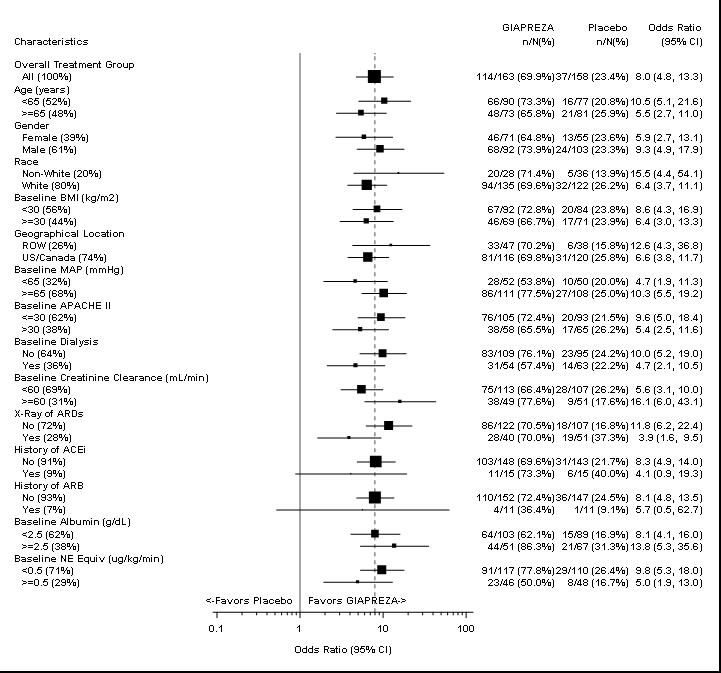
NE Equiv = norepinephrine equivalent dose: the sum of all vasopressor doses with each vasopressor dose converted to the clinically equivalent norepinephrine dose.
Note: The figure above presents effects in various subgroups, all of which are baseline characteristics. The 95% confidence limits that are shown do not take into account the number of comparisons made and may not reflect the effect of a particular factor after adjustment for all other factors. Apparent homogeneity or heterogeneity among groups should not be over-interpreted.
In the GIAPREZA-treated group, the median time to reach the target MAP endpoint was 5 minutes. The effect on MAP was sustained for at least the first three hours of treatment. The median dose of GIAPREZA was 10 ng/kg/min at 30 minutes. Of the 114 responders at Hour 3, only 2 (1.8%) received more than 80 ng/kg/min.
Patients were not necessarily on maximum doses of other vasopressors at the time of randomization. The effect of GIAPREZA when added to maximum doses of other vasopressors is unknown.
Mortality through Day 28 was 46% on GIAPREZA and 54% on placebo (hazard ratio 0.78; 95% confidence interval 0.57 – 1.07).

Dilute GIAPREZA in 0.9% sodium chloride prior to use. See Full Prescribing Information for instructions on preparation and administration of injection. Diluted solution may be stored at room temperature or under refrigeration and should be discarded after 24 hours. GIAPREZA must be administered as an intravenous infusion. (
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Discard vial and any unused portion of the drug product after use.
Diluted solution may be stored at room temperature (20°C to 25°C [68°F to 77°F]) or under refrigeration (2°C to 8°C [36°F to 46°F]). Discard prepared solution after 24 hours at room temperature or under refrigeration.
- Start GIAPREZA intravenously at 20 nanograms (ng)/kg/min. Titrate as frequently as every 5 minutes by increments of up to 15 ng/kg/min as needed. During the first 3 hours, the maximum dose should not exceed 80 ng/kg/min. Maintenance dose should not exceed 40 ng/kg/min. Doses as low as 1.25 ng/kg/min may be used.( )
2.2. AdministrationThe recommended starting dosage of GIAPREZA is 20 nanograms (ng)/kg/min via continuous intravenous infusion. Administration through a central venous line is recommended.
Monitor blood pressure response and titrate GIAPREZA as frequently as every 5 minutes by increments of up to 15 ng/kg/min as needed to achieve or maintain target blood pressure. Do not exceed 80 ng/kg/min during the first 3 hours of treatment. Maintenance dose should not exceed 40 ng/kg/min. Doses as low as 1.25 ng/kg/min may be used.
Once the underlying shock has sufficiently improved, down-titrate every 5 to 15 minutes by increments of up to 15 ng/kg/min based on blood pressure.
Injection: 0.5 mg/mL angiotensin II and 2.5 mg/mL angiotensin II in a vial.
GIAPREZA is a clear, aqueous solution.
The published data on angiotensin II use in pregnant women are not sufficient to determine a drug-associated risk of adverse developmental outcomes. Animal reproduction studies have not been conducted with GIAPREZA.
All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
None.