Get your patient on Gliadel (Carmustine)
Gliadel prior authorization resources
Most recent state uniform prior authorization forms
Dosage & administration
DOSAGE AND ADMINISTRATION
Recommended Dose
The recommended dose of GLIADEL Wafer is eight 7.7 mg wafers for a total of 61.6 mg implanted intracranially. The safety and effectiveness of repeat administration have not been studied.
Insertion Instructions
Following maximal tumor resection, confirmation of tumor pathology and establishment of hemostasis, place up to a maximum of eight GLIADEL Wafers to cover as much of the resection cavity as possible. Should the size and shape of the resected cavity not accommodate eight wafers, place the maximum number of wafers feasible within the cavity. Slight overlapping of the wafers is acceptable. Wafers broken in half may be used, but discard wafers broken in more than two pieces. Oxidized regenerated cellulose (Surgicel ® ) may be placed over the wafers to secure them against the cavity surface. After placement of the wafers, irrigate the resection cavity and close the dura in a water-tight fashion.
Preparation and Safe Handling
GLIADEL Wafers contain a cytotoxic drug. Follow applicable special handling and disposal procedures. 1
Each wafer is packaged within two nested aluminum foil laminate pouches. The inner pouch is sterile and is designed to maintain product sterility and protect the product from moisture. The outside surface of the outer laminated aluminum foil pouch is a peelable overwrap and is not sterile.
Deliver GLIADEL Wafers to the operating room in their outer aluminum foil pouch, unopened. Do not open the pouch until the wafers are ready to be implanted . GLIADEL Wafers in unopened outer foil pouches are stable at room temperature for six hours at a time for up to three cycles within a 30-day period.
Exposure to carmustine can cause severe burning and hyperpigmentation of the skin. Use double gloves when handling GLIADEL Wafers. Discard the outer gloves into a biohazard waste container after use. Use a dedicated surgical instrument for wafer implantation. If repeat neurosurgical intervention is indicated, handle residual wafers or wafer remnants as potential cytotoxic agents.
Instructions for Opening Pouch Containing GLIADEL Wafer
Read all steps of the instructions prior to opening the pouch.
Instructions for opening the pouch containing GLIADEL Wafer can be viewed at the following website: http://gliadel.com/hcp/pouch-opening-instructions . Illustrations are also pictured below.
Figure 1: To remove the sterile inner pouch from the outer pouch, locate the folded corner and slowly pull in an outward motion.

Figure 2: Do NOT pull in a downward motion rolling knuckles over the pouch. This may exert pressure on the wafer and cause it to break.

Figure 3: The inner pouch is a multi-layered, silver colored, foil laminate. Remove the inner pouch by grabbing hold of the crimped edge of the inner pouch using a sterile instrument and pulling upward.

Figure 4: To open the inner pouch, gently hold the crimped edge and cut in an arc-like fashion around the wafer.

Figure 5: To remove the GLIADEL Wafer, gently grasp the wafer with the aid of forceps and place it onto a designated sterile field.


By using PrescriberAI, you agree to the AI Terms of Use.
Gliadel prescribing information
INDICATIONS AND USAGE
GLIADEL Wafer is indicated for the treatment of patients with:
- newly-diagnosed high-grade glioma as an adjunct to surgery and radiation, and
- recurrent glioblastoma as an adjunct to surgery.
DOSAGE AND ADMINISTRATION
Recommended Dose
The recommended dose of GLIADEL Wafer is eight 7.7 mg wafers for a total of 61.6 mg implanted intracranially. The safety and effectiveness of repeat administration have not been studied.
Insertion Instructions
Following maximal tumor resection, confirmation of tumor pathology and establishment of hemostasis, place up to a maximum of eight GLIADEL Wafers to cover as much of the resection cavity as possible. Should the size and shape of the resected cavity not accommodate eight wafers, place the maximum number of wafers feasible within the cavity. Slight overlapping of the wafers is acceptable. Wafers broken in half may be used, but discard wafers broken in more than two pieces. Oxidized regenerated cellulose (Surgicel ® ) may be placed over the wafers to secure them against the cavity surface. After placement of the wafers, irrigate the resection cavity and close the dura in a water-tight fashion.
Preparation and Safe Handling
GLIADEL Wafers contain a cytotoxic drug. Follow applicable special handling and disposal procedures. 1
Each wafer is packaged within two nested aluminum foil laminate pouches. The inner pouch is sterile and is designed to maintain product sterility and protect the product from moisture. The outside surface of the outer laminated aluminum foil pouch is a peelable overwrap and is not sterile.
Deliver GLIADEL Wafers to the operating room in their outer aluminum foil pouch, unopened. Do not open the pouch until the wafers are ready to be implanted . GLIADEL Wafers in unopened outer foil pouches are stable at room temperature for six hours at a time for up to three cycles within a 30-day period.
Exposure to carmustine can cause severe burning and hyperpigmentation of the skin. Use double gloves when handling GLIADEL Wafers. Discard the outer gloves into a biohazard waste container after use. Use a dedicated surgical instrument for wafer implantation. If repeat neurosurgical intervention is indicated, handle residual wafers or wafer remnants as potential cytotoxic agents.
Instructions for Opening Pouch Containing GLIADEL Wafer
Read all steps of the instructions prior to opening the pouch.
Instructions for opening the pouch containing GLIADEL Wafer can be viewed at the following website: http://gliadel.com/hcp/pouch-opening-instructions . Illustrations are also pictured below.
Figure 1: To remove the sterile inner pouch from the outer pouch, locate the folded corner and slowly pull in an outward motion.

Figure 2: Do NOT pull in a downward motion rolling knuckles over the pouch. This may exert pressure on the wafer and cause it to break.

Figure 3: The inner pouch is a multi-layered, silver colored, foil laminate. Remove the inner pouch by grabbing hold of the crimped edge of the inner pouch using a sterile instrument and pulling upward.

Figure 4: To open the inner pouch, gently hold the crimped edge and cut in an arc-like fashion around the wafer.

Figure 5: To remove the GLIADEL Wafer, gently grasp the wafer with the aid of forceps and place it onto a designated sterile field.

DOSAGE FORMS AND STRENGTHS
GLIADEL Wafer is an off-white to pale yellow round wafer. Each GLIADEL Wafer contains 7.7 mg of carmustine.
USE IN SPECIFIC POPULATIONS
- Lactation: Advise not to breastfeed (8.2 ).
Pregnancy
Risk Summary
GLIADEL Wafer can cause fetal harm when administered to a pregnant woman. There are no available data on GLIADEL use in pregnant women. There have been no animal reproductive studies with GLIADEL Wafer; however, carmustine, the active component of GLIADEL Wafer, is embryotoxic and teratogenic in rats at exposures less than the exposure at the recommended human dose based on body surface area (BSA) and embryotoxic in rabbits at exposures similar to exposures at the recommended human dose based on BSA (see Data ) . Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
There are no studies assessing the reproductive toxicity of GLIADEL Wafer; however, carmustine, the active component of GLIADEL Wafer, is embryotoxic and teratogenic in rats at intraperitoneal doses of 0.5 mg/kg/day or greater when given on gestation days 6 through 15. Carmustine caused fetal malformations (anophthalmia, micrognathia, omphalocele) at 1 mg/kg/day (about 0.12 times the recommended human dose, eight wafers of 7.7 mg carmustine/wafer, based on BSA). Carmustine was embryotoxic in rabbits at intravenous doses of 4 mg/kg/day (about 1.2 times the recommended human dose based on BSA). Embryotoxicity was characterized by increased embryo-fetal deaths, reduced numbers of litters, and reduced litter sizes.
Lactation
Risk Summary
No data are available regarding the presence of carmustine, the active component of GLIADEL Wafer, or its metabolites in human milk or its effects on the breastfed child or on milk production. Because of the potential for serious adverse reactions in breastfed children from GLIADEL Wafers, advise women not to breastfeed following implantation with GLIADEL Wafers and for at least 7 days after implantation.
Females and Males of Reproductive Potential
Pregnancy Testing
Verify pregnancy status of females of reproductive potential prior to implantation with GLIADEL Wafer [see Use in Specific Populations (8.1) ].
Contraception
GLIADEL Wafer can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1) ] .
Females Advise females of reproductive potential to use effective contraception for 6 months after implantation of GLIADEL Wafer.
Males
Based on its mechanism of action, advise males with female partners of reproductive potential to use effective contraception for 3 months following implantation of GLIADEL Wafer [see Clinical Pharmacology (12.1) , Nonclinical Toxicology (13.1) ].
Infertility
Males
Carmustine caused testicular degeneration in animals. Advise male patients of the potential risk of infertility [see Nonclinical Toxicology (13.1) ] .
Pediatric Use
The safety and effectiveness of GLIADEL Wafer in pediatric patients have not been established.
Geriatric Use
Clinical trials of GLIADEL Wafer did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently from younger patients.
CONTRAINDICATIONS
None.
WARNINGS AND PRECAUTIONS
- Seizures: Monitor patients for seizures following implantation (5.1 ).
- Intracranial hypertension: Monitor patients for signs of increased intracranial pressure (5.2 ).
- Impaired neurosurgical wound healing: Monitor patients for complications of craniotomy (5.3 ).
- Meningitis: Monitor patients for signs of bacterial or chemical meningitis (5.4 ).
- Wafer migration: Monitor patients for signs of obstructive hydrocephalus (5.5 ).
- Embryo-fetal toxicity: Can cause fetal harm. Advise patients of the potential risk to a fetus. Advise males and females of reproductive potential to use an effective method of contraception. (5.6 , 8.1 , 8.3 ).
Seizures
Seizures occurred in 37% of patients treated with GLIADEL Wafers for recurrent glioma in Study 2. New or worsening (treatment emergent) seizures occurred in 20% of patients; 54% of treatment emergent seizures occurred within the first 5 post-operative days [see Adverse Reactions (6.1) ]. The median time to onset of the first new or worsened post-operative seizure was four days. Institute optimal anti-seizure therapy prior to surgery. Monitor patients for seizures postoperatively.
Intracranial Hypertension
Brain edema occurred in 23% of patients with newly diagnosed glioma treated with GLIADEL Wafers in Study 1. Additionally, one GLIADEL-treated patient experienced intracerebral mass effect unresponsive to corticosteroids which led to brain herniation [see Adverse Reactions (6.1) ] . Monitor patients closely for intracranial hypertension related to brain edema, inflammation, or necrosis of the brain tissue surrounding the resection. In refractory cases, consider re-operation and removal of GLIADEL Wafers or Wafer remnants.
Impaired Neurosurgical Wound Healing
Impaired neurosurgical wound healing including wound dehiscence, delayed wound healing, and subdural, subgaleal, or wound effusions occur with GLIADEL Wafer treatment. In Study 1, 16% of GLIADEL Wafer-treated patients with newly diagnosed glioma experienced impaired intracranial wound healing and 5% had cerebrospinal fluid leaks. In Study 2, 14% of GLIADEL Wafer-treated patients with recurrent high-grade glioma experienced wound healing abnormalities [see Adverse Reactions (6.1) ]. Monitor patients post-operatively for impaired neurosurgical wound healing.
Meningitis
Meningitis occurred in 4% of patients with recurrent glioma receiving GLIADEL Wafers in Study 2. Two cases of meningitis were bacterial; one patient required removal of the Wafers four days after implantation; the other developed meningitis following reoperation for recurrent tumor. One case was diagnosed as chemical meningitis and resolved following steroid treatment. In one case the cause was unspecified, but meningitis resolved following antibiotic treatment. Monitor postoperatively for signs of meningitis and central nervous system infection.
Wafer Migration
GLIADEL Wafer migration can occur. To reduce the risk of obstructive hydrocephalus due to wafer migration into the ventricular system, close any communication larger than the diameter of a Wafer between the surgical resection cavity and the ventricular system prior to Wafer implantation. Monitor patients for signs of obstructive hydrocephalus.
Embryo-Fetal Toxicity
GLIADEL Wafers can cause fetal harm when administered to a pregnant woman. Carmustine, the active component of GLIADEL Wafer, is embryotoxic and teratogenic in rats at exposures less than the exposure at the recommended human dose based on body surface area (BSA) and embryotoxic in rabbits at exposures similar to the exposure at the recommended human dose based on BSA.
Advise patients of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception for 6 months after implantation of GLIADEL Wafer. Advise males with female partners of reproductive potential to use effective contraception for 3 months following implantation of GLIADEL Wafers [see Use in Specific Populations (8.1 , 8.3) , Nonclinical Toxicology (13.1) ].
ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling:
- Seizures [see Warnings and Precautions (5.1) ]
- Intracranial Hypertension [see Warnings and Precautions (5.2) ]
- Impaired Neurosurgical Wound Healing [see Warnings and Precautions (5.3) ]
- Meningitis [see Warnings and Precautions (5.4) ]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Newly-Diagnosed High-Grade Glioma
The safety of GLIADEL Wafers was evaluated in a multicenter, randomized (1:1), double-blind, placebo controlled trial of 240 adult patients with newly-diagnosed high-grade glioma who received up to eight GLIADEL Wafers or matched placebo implanted against the resection surfaces after maximal tumor resection (Study 1).
The population in Study 1 was 67% male and 97% White, and the median age was 53 years (range: 21-72). Eighty-seven percent had a Karnofsky performance status ≥ 70 and 71% had a Karnofsky performance status of ≥ 80%. Seventy-eight percent had a histologic subtype of glioblastoma as determined by central pathology review. Thirty-eight percent of patients received 8 wafers and 78% received ≥ 6 wafers. Starting three weeks after surgery, 80% of patients received standard limited field radiation therapy (RT) described as 55-60 Gy delivered in 28 to 30 fractions over six weeks; an additional 11% received no radiotherapy and the remainder received non-standard radiotherapy or a combination of standard and non-standard radiotherapy. At the time of progression, 12% received systemic chemotherapy.
Deaths occurred within 30 days of wafer implantation in 5 (4%) of patients receiving GLIADEL Wafers compared to 2 (2%) of patients receiving placebo. Deaths on the GLIADEL arm resulted from cerebral hematoma/edema (n=3), pulmonary embolism (n=1) and acute coronary event (n=1). Deaths on the placebo arm resulted from sepsis (n=1) and malignant disease (n=1).
The incidence of common adverse reactions in GLIADEL Wafer-treated patients is listed in Table 1. The incidence of local adverse reactions is shown in Table 2.
| Adverse Reaction | GLIADEL Wafer N=120 | Placebo N=120 |
|---|---|---|
| % | % | |
| GASTROINTESTINAL | ||
| Nausea | 22 | 17 |
| Vomiting | 21 | 16 |
| Constipation | 19 | 12 |
| Abdominal pain | 8 | 2 |
| GENERAL AND ADMINISTRATION SITE CONDITION | ||
| Asthenia | 22 | 15 |
| Chest pain | 5 | 0 |
| INJURY, POISONING AND PROCEDURAL COMPLICATIONS | ||
| Wound healing abnormalities Included (1) fluid, CDS, or subdural fluid collection; (2) CSF leak; (3) wound dehiscence, breakdown, or poor healing; and (4) subgaleal or wound effusions (including yellow discharge at the incision) | 16 | 12 |
| MUSCULOSKELETAL AND CONNECTIVE TISSUE | ||
| Back pain | 7 | 3 |
| PSYCHIATRIC | ||
| Depression | 16 | 10 |
| Local Adverse Reactions | GLIADEL Wafer N=120 | Placebo N=120 |
|---|---|---|
| % | % | |
| Cerebral edema | 23 | 19 |
| Intracranial hypertension | 9 | 2 |
| Cerebral hemorrhage | 6 | 4 |
| Brain abscess | 6 | 4 |
| Brain cyst | 2 | 3 |
Recurrent High-Grade Glioma
The safety of GLIADEL Wafers was evaluated in a multicenter, randomized (1:1), double-blind, placebo controlled trial of 222 patients with recurrent high-grade glioma who received up to eight GLIADEL Wafers or matched placebo implanted against the resection surfaces after maximal tumor resection (Study 2). Patients were required to have had prior definitive external beam radiation therapy sufficient to disqualify them from additional radiation therapy. All patients were eligible to receive chemotherapy which was withheld at least four weeks (six weeks for nitrosoureas) prior to and two weeks after surgery.
The population in Study 2 was 64% male, 92% White, and the median age was 49 years (range: 19-80). Sixty-five percent had a histologic subtype of glioblastoma, 26% had anaplastic astrocytoma or another anaplastic variant, 73% had a Karnofsky performance status ≥ 70, 53% had a Karnofsky performance status of ≥ 80%, 73% had only one prior surgery, and 46% had prior treatment with nitrosourea. Eighty-one percent of patients received 8 wafers and 96% received ≥ 6 wafers.
Sixty-four severe adverse reactions were reported in 43(39%) patients receiving GLIADEL Wafers. Adverse reactions in GLIADEL Wafer-treated patients are shown in Table 3. Meningitis occurred in four patients receiving GLIADEL Wafers and in no patients receiving placebo. Bacterial meningitis was confirmed in two patients: the first with onset four days following GLIADEL Wafer implantation; the second following resection for tumor recurrence 155 days following GLIADEL Wafer implantation. One case, attributed to chemical meningitis resolved following steroid treatment. The cause of the fourth case was undetermined but resolved following antibiotic treatment.
| Adverse Reaction | GLIADEL Wafer N=110 | Placebo N=112 |
|---|---|---|
| % | % | |
| GENERAL | ||
| Fever | 12 | 8 |
| INFECTIOUS | ||
| Urinary tract infections | 21 | 17 |
| INJURY, POISONING AND PROCEDURAL COMPLICATIONS | ||
| Wound healing abnormalities Included (1) fluid, CDS, or subdural fluid collection; (2) CSF leak; (3) wound dehiscence, breakdown, or poor healing; and (4) subgaleal or wound effusions (including yellow discharge at the incision) | 14 | 5 |
The incidence of seizures is shown in Table 4. The incidence of hydrocephalus, cerebral edema and intracranial hypertension is shown in Table 5.
| Adverse Reaction | GLIADEL Wafer N=110 | Placebo N=112 |
|---|---|---|
| Patients with seizures (%) | ||
| Any seizures after wafer implantation | 37 | 29 |
| New or worsening seizures | 20 | 20 |
| Time to new or worsening seizures (days) Days from implantation to onset of first new or worsening seizure. | ||
| Mean (SD) | 26.09 (0.75) | 62.36 (48.66) |
| Median | 3.5 | 61.0 |
| Adverse Reaction | GLIADEL Wafer N=110 | Placebo N=112 |
|---|---|---|
| % | % | |
| Hydrocephalus | 5 | 2 |
| Cerebral edema | 4 | 1 |
DESCRIPTION
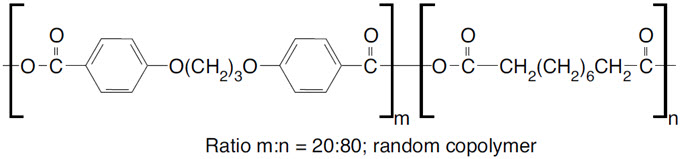
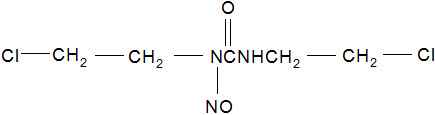
GLIADEL Wafer is an implant for intracranial use, containing carmustine, a nitrosourea alkylating agent, and polifeprosan, a biodegradable copolymer used to control the release of carmustine. It is a sterile, off-white to pale yellow wafer approximately 1.45 cm in diameter and 1 mm thick. Each wafer contains 7.7 mg of carmustine [1, 3-bis (2-chloroethyl)-1-nitrosourea, or BCNU] and 192.3 mg of a biodegradable polyanhydride copolymer. The copolymer, polifeprosan 20, consists of poly [bis (p-carboxyphenoxy)] propane and sebacic acid in a 20:80 molar ratio. Carmustine is homogeneously distributed in the copolymer matrix.
The structural formula for polifeprosan 20 is:
The structural formula for carmustine is:
CLINICAL PHARMACOLOGY
Mechanism of Action
The activity of GLIADEL Wafer is due to release of cytotoxic concentrations of carmustine, a DNA and RNA alkylating agent, into the tumor resection cavity. On exposure to the aqueous environment of the resection cavity, the anhydride bonds in the copolymer are hydrolyzed, releasing carmustine, carboxyphenoxypropane, and sebacic acid into the surrounding brain tissue.
Pharmacokinetics
Carmustine concentrations delivered by GLIADEL Wafer in human brain tissue have not been determined. Following wafer insertion, the mean whole blood C max (± SD) is 10.2 ng/mL ± 4.8 ng/mL.
Absorption
Systemic absorption of carmustine is measurable for approximately 24 hours after wafer insertion. Carmustine C max was reached approximately 3 hours after wafer insertion.
Elimination
Metabolism
Carmustine degrades both spontaneously and metabolically.
Wafer Biodegradation
GLIADEL Wafers are biodegradable when implanted into the human brain. Wafer remnants were visible on CT scans obtained 49 days after implantation of GLIADEL Wafer. More than 70% of the copolymer degrades within three weeks. Wafer remnants have been present at re-operation and autopsy up to 7.8 months after GLIADEL Wafer implantation and consisted mostly of water and monomeric components with minimal detectable carmustine present.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity, mutagenicity, or impairment of fertility studies have been conducted with GLIADEL Wafer. Carcinogenicity, mutagenicity, and impairment of fertility studies have been conducted with carmustine, the active component of GLIADEL Wafer. Carmustine was carcinogenic in rats and mice when delivered by intraperitoneal injection at doses lower than those delivered by GLIADEL Wafer at the recommended dose. There were increases in tumor incidence in all treated animals. Carmustine was mutagenic in vitro (Ames assay, human lymphoblast HGPRT assay) and clastogenic both in vitro (V79 hamster cell micronucleus assay) and in vivo (SCE assay in rodent brain tumors, mouse bone marrow micronucleus assay).
In male rats carmustine caused testicular degeneration at intraperitoneal doses of 8 mg/kg/week for eight weeks (about 1.3 times the recommended human dose based on body surface area).
CLINICAL STUDIES
Newly-Diagnosed High-Grade Glioma
Study 1 was a multicenter, double-blind, placebo-controlled, clinical trial in adult patients with newly-diagnosed high-grade glioma. A total of 240 patients were randomized (1:1) to receive up to eight GLIADEL Wafers or matched placebo wafers following maximal tumor resection. Patients received post-operative radiation therapy (55-60 Gy delivered in 28 to 30 fractions over six weeks) starting three weeks after surgery. Patients with anaplastic oligodendroglioma also received systemic chemotherapy (6 cycles of PCV- lomustine 110 mg/m 2 day 1, procarbazine 60 mg/m 2 days 8-21, vincristine 1.4 mg/m 2 days 8 and 29).
The population in Study 1 was 67% male and 97% White, and the median age was 53 years (range: 21-72). Eighty-seven percent had a Karnofsky performance status ≥ 70% and 71% had a Karnofsky performance status of ≥ 80%. Seventy-eight percent had a histologic subtype of glioblastoma as determined by central pathology review. Thirty-eight percent of patients received 8 wafers and 78% received ≥ 6 wafers. Starting three weeks after surgery, 80% of patients received standard limited field radiation therapy (RT) described as 55-60 Gy delivered in 28 to 30 fractions over six weeks; 11% received no radiotherapy and the remainder received non-standard radiotherapy or a combination of standard and non-standard radiotherapy. At the time of progression, 12% received systemic chemotherapy. Patients were followed for at least three years or until death.
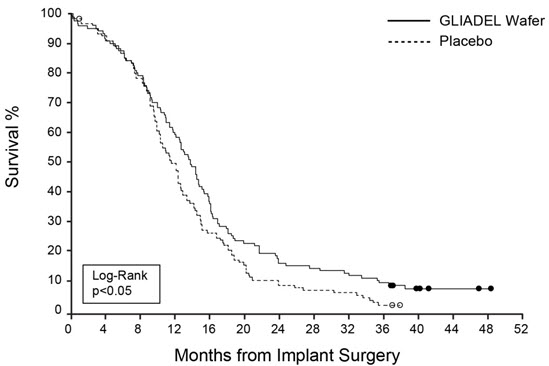
Efficacy results for patients randomized in Study 1 are summarized in Table 6 and Figure 6. Overall survival among all patients with newly diagnosed high-grade glioma, the primary outcome measure, was prolonged in the GLIADEL arm. Overall survival in the subset of patients with glioblastoma, a secondary outcome measure, was not significantly prolonged.
| Overall Survival – ITT Based on a post-final analysis, protocol specified non-stratified log-rank test. | Gliadel Wafer (n=120) | Placebo Wafer (n=120) |
|---|---|---|
| Number of deaths, n (%) | 111 (93%) | 117(98%) |
| Median overall survival, months (95% CI) | 13.9 (12.1, 15.1) | 11.6 (10.2, 12.7) |
| Hazard ratio (95% CI) | 0.73 (0.56, 0.95) | |
| Log-Rank test p-value | <0.02 p-value not adjusted for multiple comparisons | |
Figure 6: Kaplan-Meier Curves of Overall Survival in Patients with Newly Diagnosed High-Grade Glioma, Study 1. Based on a post-final analysis, protocol specified non-stratified log-rank test; p-value not adjusted for multiple comparisons
Recurrent Glioblastoma
Study 2 was a multicenter, double-blind, placebo controlled, clinical trial in adult patients with recurrent high-grade glioma. Patients were required to have had prior definitive external beam radiation therapy sufficient to disqualify them from additional radiation therapy. Following maximal tumor resection and confirmation of high-grade glioma, a total of 222 patients were randomized (1:1) to receive a maximum of eight GLIADEL Wafers (n=110) or matched placebo wafers (n=112) positioned to cover the entire resection surface. All patients were eligible to receive chemotherapy which was withheld at least four weeks (six weeks for nitrosoureas) prior to and two weeks after surgery. Patients were followed for up to 71 months.
The population in Study 2 was 64% male and 92% White, and the median age was 49 years (range: 19-80). Sixty-five percent had a histologic subtype of glioblastoma, 26% had anaplastic astrocytoma or another anaplastic variant, 73% had a Karnofsky performance status ≥ 70, 53% had a Karnofsky performance status of ≥ 80%, 73% had only one prior surgery, and 46% had prior treatment with nitrosourea. Eighty-one percent of patients received 8 wafers and 96% received ≥ 6 wafers.
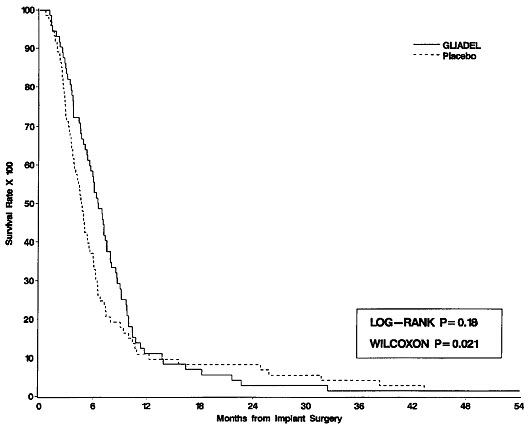
Survival and 6-month mortality rate in the subgroup of patients with recurrent glioblastoma, were exploratory outcome measures and are summarized in Table 7 and Figures 7 and 8. No survival prolongation was observed in patients with pathologic diagnoses other than glioblastoma.
| GLIADEL Wafer | Placebo Wafer | |
|---|---|---|
| GLIOBLASTOMA | n=72 | n=73 |
| 6-Month Survival | ||
| Number of deaths, n (%) | 32 | 47 |
| 6-month survival rate (%) | 56% | 36% |
| Log-Rank test p-value Gehan's generalized Wilcoxon Test p-value | 0.013 p-value not adjusted for multiple comparisons 0.015 | |
| Overall Survival | ||
| Number of deaths, n (%) | 71 (99%) | 72 (99%) |
| Median overall survival (95% CI (months) | 6.51 (5.32, 7.49) | 4.63 (3.78, 5.52) |
| Log-Rank test p-value Gehan's generalized Wilcoxon Test p-value | 0.181 0.021 | |
Figure 7: Kaplan-Meier Curves of 6-Month Survival for Patients with Recurrent Glioblastoma, Study 2.
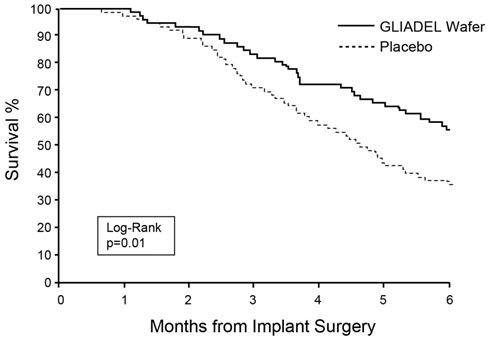
Figure 8: Kaplan-Meier Curves of Overall Survival for Patients with Recurrent Glioblastoma, Study 2.
HOW SUPPLIED/STORAGE AND HANDLING
GLIADEL Wafer is supplied in a single dose treatment box containing eight individually pouched wafers. Each wafer contains 7.7 mg of carmustine and is packaged in two aluminum foil laminate pouches. The inner pouch is sterile and is designed to maintain product sterility and protect the product from moisture. The outer pouch is a peelable overwrap. The outside surface of the outer pouch is not sterile.
NDC for single dose treatment box: 24338-050-08
Store GLIADEL Wafer at or below -20ºC (-4ºF).
Do not keep unopened foil pouches at ambient room temperature for more than six hours at a time for up to three cycles within a 30-day period.
GLIADEL Wafer is a cytotoxic drug and special handling and disposal procedures should be considered. 1
Mechanism of Action
The activity of GLIADEL Wafer is due to release of cytotoxic concentrations of carmustine, a DNA and RNA alkylating agent, into the tumor resection cavity. On exposure to the aqueous environment of the resection cavity, the anhydride bonds in the copolymer are hydrolyzed, releasing carmustine, carboxyphenoxypropane, and sebacic acid into the surrounding brain tissue.