Get your patient on Humalog 50/50 (Insulin Lispro)
Humalog 50/50 prior authorization resources
Most recent Humalog 50/50 prior authorization forms
Most recent state uniform prior authorization forms
Humalog 50/50 patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
- See Full Prescribing Information for important preparation and administration instructions. (2.1 )
- Inject HUMALOG Mix50/50 subcutaneously (within 15 minutes before a meal) in abdominal wall, thigh, upper arm, or buttocks and rotate injection sites to reduce the risk of lipodystrophy and localized cutaneous amyloidosis. (2.1 , 2.2 )
- Individualize and adjust dosage based on metabolic needs, blood glucose monitoring results and glycemic control goal. (2.2 )
- Do not administer HUMALOG Mix50/50 intravenously or by a continuous subcutaneous insulin infusion pump. (2.1 )
- HUMALOG Mix50/50 is typically dosed twice daily (with each dose intended to cover 2 meals or a meal and a snack). (2.2 )
Important Preparation and Administration Instructions
Preparation Instructions
- Always check insulin labels before administration [see Warnings and Precautions (5.4 )] .
- HUMALOG Mix50/50 is a suspension that must be resuspended immediately before use. Resuspension is easier when the insulin has reached room temperature.
- To resuspend the
- vial, carefully invert the vial at least 10 times until the suspension appears uniformly white and cloudy.
- KwikPen, gently roll the KwikPen at least 10 times and then carefully invert the KwikPen at least 10 times until the suspension appears uniformly white and cloudy.
- Inspect HUMALOG Mix50/50 visually before use. Do not use if discoloration or particulate matter is seen.
Administration Instructions
- After resuspension, immediately administer HUMALOG Mix50/50 by subcutaneous injection into the abdominal wall, thigh, upper arm, or buttocks.
- Rotate the injection site within the same region from one injection to the next to reduce the risk of lipodystrophy and localized cutaneous amyloidosis. Do not inject into areas of lipodystrophy or localized cutaneous amyloidosis [see Warnings and Precautions (5.2 ) and Adverse Reactions (6 )] .
- The HUMALOG Mix50/50 KwikPen dials in 1 unit increments.
- Use HUMALOG Mix50/50 KwikPen with caution in patients with visual impairment that may rely on audible clicks to dial their dose.
- Do not administer HUMALOG Mix50/50 intravenously or by a continuous subcutaneous insulin infusion pump.
- Do not mix HUMALOG Mix50/50 with any other insulins or diluents.
Dosage Recommendations
- Individualize and adjust the dosage of HUMALOG Mix50/50 based on the individual's metabolic needs, blood glucose monitoring results and glycemic control goal.
- Inject HUMALOG Mix50/50 subcutaneously within 15 minutes before a meal.
- HUMALOG Mix50/50 is typically dosed twice-daily (with each dose intended to cover 2 meals or a meal and a snack).
- Dosage adjustments may be needed with changes in physical activity, changes in meal patterns (i.e., macronutrient content or timing of food intake), changes in renal or hepatic function or during acute illness [see Warnings and Precautions (5.2 , 5.3 ) and Use in Specific Populations (8.6 , 8.7 )] .
- When switching from another insulin to HUMALOG Mix50/50, a different dosage of HUMALOG Mix50/50 may be needed.
- During changes to a patient's insulin regimen, increase the frequency of blood glucose monitoring [see Warnings and Precautions (5.2 )] .
Dosage Modifications for Drug Interactions
Dosage modification may be needed when HUMALOG Mix50/50 is used concomitantly with certain drugs [see Drug Interactions (7 )] .

By using PrescriberAI, you agree to the AI Terms of Use.
Humalog 50/50 prescribing information
INDICATIONS AND USAGE
HUMALOG Mix50/50 is indicated to improve glycemic control in adults with diabetes mellitus.
Limitations of Use
The proportions of rapid-acting and intermediate-acting insulins in HUMALOG Mix50/50 are fixed and do not allow for basal versus prandial dose adjustments.
DOSAGE AND ADMINISTRATION
- See Full Prescribing Information for important preparation and administration instructions. (2.1 )
- Inject HUMALOG Mix50/50 subcutaneously (within 15 minutes before a meal) in abdominal wall, thigh, upper arm, or buttocks and rotate injection sites to reduce the risk of lipodystrophy and localized cutaneous amyloidosis. (2.1 , 2.2 )
- Individualize and adjust dosage based on metabolic needs, blood glucose monitoring results and glycemic control goal. (2.2 )
- Do not administer HUMALOG Mix50/50 intravenously or by a continuous subcutaneous insulin infusion pump. (2.1 )
- HUMALOG Mix50/50 is typically dosed twice daily (with each dose intended to cover 2 meals or a meal and a snack). (2.2 )
Important Preparation and Administration Instructions
Preparation Instructions
- Always check insulin labels before administration [see Warnings and Precautions (5.4 )] .
- HUMALOG Mix50/50 is a suspension that must be resuspended immediately before use. Resuspension is easier when the insulin has reached room temperature.
- To resuspend the
- vial, carefully invert the vial at least 10 times until the suspension appears uniformly white and cloudy.
- KwikPen, gently roll the KwikPen at least 10 times and then carefully invert the KwikPen at least 10 times until the suspension appears uniformly white and cloudy.
- Inspect HUMALOG Mix50/50 visually before use. Do not use if discoloration or particulate matter is seen.
Administration Instructions
- After resuspension, immediately administer HUMALOG Mix50/50 by subcutaneous injection into the abdominal wall, thigh, upper arm, or buttocks.
- Rotate the injection site within the same region from one injection to the next to reduce the risk of lipodystrophy and localized cutaneous amyloidosis. Do not inject into areas of lipodystrophy or localized cutaneous amyloidosis [see Warnings and Precautions (5.2 ) and Adverse Reactions (6 )] .
- The HUMALOG Mix50/50 KwikPen dials in 1 unit increments.
- Use HUMALOG Mix50/50 KwikPen with caution in patients with visual impairment that may rely on audible clicks to dial their dose.
- Do not administer HUMALOG Mix50/50 intravenously or by a continuous subcutaneous insulin infusion pump.
- Do not mix HUMALOG Mix50/50 with any other insulins or diluents.
Dosage Recommendations
- Individualize and adjust the dosage of HUMALOG Mix50/50 based on the individual's metabolic needs, blood glucose monitoring results and glycemic control goal.
- Inject HUMALOG Mix50/50 subcutaneously within 15 minutes before a meal.
- HUMALOG Mix50/50 is typically dosed twice-daily (with each dose intended to cover 2 meals or a meal and a snack).
- Dosage adjustments may be needed with changes in physical activity, changes in meal patterns (i.e., macronutrient content or timing of food intake), changes in renal or hepatic function or during acute illness [see Warnings and Precautions (5.2 , 5.3 ) and Use in Specific Populations (8.6 , 8.7 )] .
- When switching from another insulin to HUMALOG Mix50/50, a different dosage of HUMALOG Mix50/50 may be needed.
- During changes to a patient's insulin regimen, increase the frequency of blood glucose monitoring [see Warnings and Precautions (5.2 )] .
Dosage Modifications for Drug Interactions
Dosage modification may be needed when HUMALOG Mix50/50 is used concomitantly with certain drugs [see Drug Interactions (7 )] .
DOSAGE FORMS AND STRENGTHS
Injectable suspension: 100 units/mL (U-100) of HUMALOG Mix50/50, 50% insulin lispro protamine and 50% insulin lispro, is a white and cloudy suspension available as:
- 10 mL multiple-dose vial
- 3 mL single-patient-use KwikPen prefilled pen
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
Published studies with insulin lispro used during pregnancy have not reported an association between insulin lispro and the induction of major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data). There are risks to the mother and fetus associated with poorly controlled diabetes in pregnancy (see Clinical Considerations).
Pregnant rats and rabbits were exposed to insulin lispro in animal reproduction studies during organogenesis. No adverse effects on embryo/fetal viability or morphology were observed in offspring of rats exposed to insulin lispro at a dose approximately 3 times the human subcutaneous dose of 1 unit insulin lispro/kg/day. No adverse effects on embryo/fetal development were observed in offspring of rabbits exposed to insulin lispro at doses up to approximately 0.2 times the human subcutaneous dose of 1 unit/kg/day (see Data).
The estimated background risk of major birth defects is 6-10% in women with pre-gestational diabetes with a HbA1c >7 and has been reported to be as high as 20-25% in women with a HbA1c >10. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Poorly controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, pre-eclampsia, spontaneous abortions, preterm delivery, and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, still birth, and macrosomia related morbidity.
Data
Human Data
Published data from retrospective studies and meta-analyses do not report an association with insulin lispro and major birth defects, miscarriage, or adverse maternal or fetal outcomes when insulin lispro is used during pregnancy. However, these studies cannot definitely establish or exclude the absence of any risk because of methodological limitations including small sample size, selection bias, confounding by unmeasured factors, and some lacking comparator groups.
Animal Data
Animal reproduction studies have not been performed with HUMALOG Mix50/50. However, subcutaneous reproduction and teratology studies have been conducted with insulin lispro (a component of HUMALOG Mix50/50). In a combined fertility and embryo-fetal development study, female rats were given subcutaneous insulin lispro injections of 1, 5, and 20 units/kg/day (0.2, 0.8, and 3 times the human subcutaneous dose of 1 unit insulin lispro/kg/day, based on units/body surface area, respectively) from 2 weeks prior to cohabitation through Gestation Day 19. There were no adverse effects on female fertility, implantation, or fetal viability and morphology. However, fetal growth retardation was produced at the 20 units/kg/day-dose as indicated by decreased fetal weight and an increased incidence of fetal runts/litter.
In an embryo-fetal development study in pregnant rabbits, insulin lispro doses of 0.1, 0.25, and 0.75 unit/kg/day (0.03, 0.08, and 0.2 times the human subcutaneous dose of 1 unit insulin lispro/kg/day, based on units/body surface area, respectively) were injected subcutaneously on Gestation days 7 through 19. There were no adverse effects on fetal viability, weight, and morphology at any dose.
Lactation
Risk Summary
Available data from published literature suggests that exogenous human insulin products, including insulin lispro, are transferred into human milk. There are no adverse reactions reported in breastfed infants in the literature. There are no data on the effects of exogenous human insulin products, including insulin lispro, on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for insulin, any potential adverse effects on the breastfed child from HUMALOG Mix50/50 or from the underlying maternal condition.
Pediatric Use
Safety and effectiveness of HUMALOG Mix50/50 in pediatric patients have not been established.
Geriatric Use
Clinical studies of Humalog Mix50/50 did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently than younger patients. In elderly patients with diabetes, the initial dosing, dose increments, and maintenance dosage should be conservative to reduce the risk of hypoglycemia [see Warnings and Precautions (5.3 )].
Renal Impairment
The effect of renal impairment on the pharmacokinetics of HUMALOG Mix50/50 has not been studied. Patients with renal impairment may be at increased risk of hypoglycemia and may require more frequent HUMALOG Mix50/50 dose adjustment and more frequent glucose monitoring [see Warnings and Precautions (5.3 )] .
Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of HUMALOG Mix50/50 has not been studied. Patients with hepatic impairment may be at increased risk of hypoglycemia and may require more frequent HUMALOG Mix50/50 dose adjustment and more frequent glucose monitoring [see Warnings and Precautions (5.3 )] .
WARNINGS AND PRECAUTIONS
- Never share a HUMALOG Mix50/50 KwikPen or syringe between patients, even if the needle is changed. (5.1 )
- Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen: Make changes to a patient's insulin regimen (e.g., insulin strength, manufacturer, type, injection site or method of administration) under close medical supervision with increased frequency of blood glucose monitoring. (5.2 )
- Hypoglycemia: May be life-threatening. Increase frequency of blood glucose monitoring with changes to insulin dosage, co-administered glucose lowering medications, meal pattern, physical activity; in patients with renal or hepatic impairment; and in patients with hypoglycemia unawareness. (5.3 )
- Hypoglycemia Due to Medication Errors: Accidental mix-ups between insulin products can occur. Instruct patients to check insulin labels before injection. (5.4 )
- Hypersensitivity Reactions: Severe, life-threatening, generalized allergy, including anaphylaxis, can occur. Discontinue HUMALOG Mix50/50, monitor and treat if indicated. (5.5 )
- Hypokalemia: May be life-threatening. Monitor potassium levels in patients at risk of hypokalemia and treat if indicated. (5.6 )
- Fluid Retention and Heart Failure with Concomitant Use of Thiazolidinediones (TZDs) : Observe for signs and symptoms of heart failure; consider dosage reductions or discontinuation if heart failure occurs. (5.7 )
Never Share a HUMALOG Mix50/50 KwikPen or Syringe Between Patients
HUMALOG Mix50/50 KwikPens must never be shared between patients, even if the needle is changed. Patients using HUMALOG Mix50/50 vials must never share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens.
Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen
Changes in an insulin regimen (e.g., insulin strength, manufacturer, type, injection site or method of administration) may affect glycemic control and predispose to hypoglycemia [see Warnings and Precautions (5.3 )] or hyperglycemia. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis have been reported to result in hyperglycemia; and a sudden change in the injection site (to an unaffected area) has been reported to result in hypoglycemia [see Adverse Reactions (6 )] .
Make any changes to a patient's insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. Advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor for hypoglycemia. For patients with type 2 diabetes, dosage adjustments of concomitant anti-diabetic products may be needed.
Hypoglycemia
Hypoglycemia is the most common adverse reaction associated with all insulins, including HUMALOG Mix50/50. Severe hypoglycemia can cause seizures, may lead to unconsciousness, may be life-threatening, or cause death. Hypoglycemia can impair concentration ability and reaction time, this may place an individual and others at risk in situations where these abilities are important (e.g., driving or operating other machinery).
Hypoglycemia can happen suddenly, and symptoms may differ in each individual and change over time in the same individual. Symptomatic awareness of hypoglycemia may be less pronounced in patients with longstanding diabetes, in patients with diabetic nerve disease, in patients using medications that block the sympathetic nervous system (e.g., beta-blockers) [see Drug Interactions (7 )], or in patients who experience recurrent hypoglycemia.
Risk Factors for Hypoglycemia
The risk of hypoglycemia after an injection is related to the duration of action of the insulin and in general, is highest when the glucose lowering effect of the insulin is maximal. As with all insulins, the glucose lowering effect time course of HUMALOG Mix50/50 may vary in different individuals or at different times in the same individual and depends on many conditions, including the area of injection as well as the injection site blood supply and temperature [see Clinical Pharmacology (12.2 )] . Other factors which may increase the risk of hypoglycemia include changes in meal pattern (e.g., macronutrient content or timing of meals), changes in level of physical activity, or changes to co-administered medication [see Drug Interactions (7 )] . Patients with renal or hepatic impairment may be at higher risk of hypoglycemia [see Use in Specific Populations (8.6 , 8.7 )] .
Risk Mitigation Strategies for Hypoglycemia
Patients and caregivers must be educated to recognize and manage hypoglycemia. Self-monitoring of blood glucose plays an essential role in the prevention and management of hypoglycemia. In patients at higher risk for hypoglycemia and patients who have reduced symptomatic awareness of hypoglycemia, increased frequency of blood glucose monitoring is recommended.
Hypoglycemia Due to Medication Errors
Accidental mix-ups between insulin products have been reported. To avoid medication errors between HUMALOG Mix50/50 and other insulins, instruct patients to always check the insulin label before each injection.
Hypersensitivity Reactions
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulins, including HUMALOG Mix50/50. If hypersensitivity reactions occur, discontinue HUMALOG Mix50/50; treat per standard of care and monitor until symptoms and signs resolve. HUMALOG Mix50/50 is contraindicated in patients who have had hypersensitivity reactions to HUMALOG Mix50/50 or any of its excipients [see Contraindications (4 )] .
Hypokalemia
All insulins, including HUMALOG Mix50/50, cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia if indicated (e.g., patients using potassium-lowering medications, patients taking medications sensitive to serum potassium concentrations).
Fluid Retention and Heart Failure with Concomitant Use of PPAR-gamma Agonists
Thiazolidinediones (TZDs), which are peroxisome proliferator-activated receptor (PPAR)-gamma agonists, can cause dose-related fluid retention, particularly when used in combination with insulin. Fluid retention may lead to or exacerbate heart failure. Patients treated with insulin, including HUMALOG Mix50/50, and a PPAR-gamma agonist should be observed for signs and symptoms of heart failure. If heart failure develops, it should be managed according to current standards of care, and discontinuation or dose reduction of the PPAR-gamma agonist must be considered.
ADVERSE REACTIONS
The following adverse reactions are discussed elsewhere in the labeling:
Adverse Reactions from Clinical Studies or Postmarketing Reports
The following adverse reactions have been identified during post-marketing use of HUMALOG Mix50/50. Because some of these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse reactions associated with insulin initiation and glucose control intensification
Intensification or rapid improvement in glucose control has been associated with a transitory, reversible ophthalmologic refraction disorder, worsening of diabetic retinopathy, and acute painful peripheral neuropathy. Over the long-term, improved glycemic control decreases the risk of diabetic retinopathy and neuropathy.
Hypersensitivity reactions
Severe, life-threatening, generalized allergy, including anaphylaxis.
Hypoglycemia
Hypoglycemia is the most commonly observed adverse reaction in HUMALOG Mix50/50.
Hypokalemia
HUMALOG Mix50/50 can cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia.
Injection site reactions
HUMALOG Mix50/50 can cause local injection site reactions including redness, swelling, or itching at the site of injection. These reactions usually resolve in a few days to a few weeks, but in some occasions, may require discontinuation. Localized reactions and generalized myalgias have been reported with the use of meta-cresol, which is an excipient in HUMALOG Mix50/50.
Lipodystrophy
Administration of insulin subcutaneously, including HUMALOG Mix50/50, has resulted in lipoatrophy (depression in the skin) or lipohypertrophy (enlargement or thickening of tissue) [see Dosage and Administration (2.1 )] in some patients.
Localized cutaneous amyloidosis
Localized cutaneous amyloidosis at the injection site has occurred. Hyperglycemia has been reported with repeated insulin injections into areas of localized cutaneous amyloidosis; hypoglycemia has been reported with a sudden change to an unaffected injection site.
Medication Errors
Medication errors in which other insulins have been accidentally substituted for HUMALOG Mix50/50 have been identified during postapproval use.
Peripheral Edema
Insulins, including HUMALOG Mix50/50, may cause sodium retention and edema, particularly if previously poor metabolic control is improved by intensified insulin therapy.
Weight gain
Weight gain can occur with insulins, including HUMALOG Mix50/50, and has been attributed to the anabolic effects of insulin and the decrease in glycosuria.
Immunogenicity
As with all therapeutic proteins, insulin administration may cause anti-insulin antibodies to form. The incidence of antibody formation with HUMALOG Mix50/50 is unknown.
DRUG INTERACTIONS
Table 1 describes the clinically significant drug interactions with HUMALOG Mix50/50.
| Drugs that May Increase the Risk of Hypoglycemia | |
| Drugs: | Antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analog (e.g., octreotide), and sulfonamide antibiotics. |
| Intervention: | Dose adjustment and increased frequency of glucose monitoring may be required when HUMALOG Mix50/50 is co-administered with these drugs. |
| Drugs that May Decrease the Blood Glucose Lowering Effect of HUMALOG Mix50/50 | |
| Drugs: | Atypical antipsychotics (e.g., olanzapine and clozapine), corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones. |
| Intervention: | Dose adjustment and increased frequency of glucose monitoring may be required when HUMALOG Mix50/50 is co-administered with these drugs. |
| Drugs that May Increase or Decrease the Blood Glucose Lowering Effect of HUMALOG Mix50/50 | |
| Drugs: | Alcohol, beta-blockers, clonidine, and lithium salts. Pentamidine may cause hypoglycemia, which may sometimes be followed by hyperglycemia. |
| Intervention: | Dose adjustment and increased frequency of glucose monitoring may be required when HUMALOG Mix50/50 is co-administered with these drugs. |
| Drugs that May Blunt Signs and Symptoms of Hypoglycemia | |
| Drugs: | Beta-blockers, clonidine, guanethidine, and reserpine. |
| Intervention: | Increased frequency of glucose monitoring may be required when HUMALOG Mix50/50 is co-administered with these drugs. |
DESCRIPTION
Insulin lispro is a rapid-acting insulin analog produced by recombinant DNA technology utilizing a non-pathogenic laboratory strain of Escherichia coli . Insulin lispro differs from human insulin in that the amino acid proline at position B28 is replaced by lysine and the lysine in position B29 is replaced by proline. Chemically, it is Lys(B28), Pro(B29) human insulin analog and has the empirical formula C 257 H 383 N 65 O 77 S 6 and a molecular weight of 5.808 kDa, both identical to that of human insulin.
Humalog Mix50/50 (insulin lispro protamine and insulin lispro) injectable suspension is a mixture of 50% insulin lispro protamine, an intermediate-acting human insulin analog, and 50% insulin lispro, a rapid-acting human insulin analog. Insulin lispro protamine suspension is a suspension of crystals produced from combining insulin lispro and protamine sulfate under appropriate conditions for crystal formation.
Insulin lispro has the following primary structure:

HUMALOG Mix50/50 (insulin lispro protamine and insulin lispro) injectable suspension is a sterile, white and cloudy suspension for subcutaneous use.
Each mL of HUMALOG Mix50/50 contains 100 units of insulin lispro; dibasic sodium phosphate (2.0 mg), glycerin (16 mg), metacresol (2.20 mg), phenol (0.89 mg), protamine sulfate (0.19 mg), zinc oxide content adjusted to provide zinc ion (0.0305 mg), and Water for Injection, USP. The pH is 7.0 to 7.8. Sodium hydroxide and/or hydrochloric acid is added during manufacture to adjust the pH.
CLINICAL PHARMACOLOGY
Mechanism of Action
The primary activity of insulin including HUMALOG Mix50/50 is the regulation of glucose metabolism. Insulins lower blood glucose by stimulating peripheral glucose uptake by skeletal muscle and fat, and by inhibiting hepatic glucose production. Insulins inhibit lipolysis and proteolysis, and enhance protein synthesis.
Pharmacodynamics
In a glucose clamp study performed in 30 healthy subjects, the onset of action and glucose-lowering activity of HUMALOG, HUMALOG Mix50/50, HUMALOG ® Mix75/25™, and insulin lispro protamine suspension (ILPS) were compared ( see Figure 1). Graphs of mean glucose infusion rate versus time showed a distinct insulin activity profile for each formulation. The rapid onset of glucose-lowering activity characteristic of HUMALOG was maintained in HUMALOG Mix50/50. The median maximum pharmacologic effect of HUMALOG Mix50/50 after administration of a 0.3 unit/kg dose to healthy subjects occurred at 2 hours (range: 1-5 hours); glucose lowering activity was detectable for a median of 22 hours (range: 11 to 22 hours), which was the end of the clamp.
Figure 1 should be considered only as a representative example since the time course of action of insulin and insulin analogs, may vary in different individuals or within the same individual.
Figure 1: Mean Insulin Activity Versus Time Profiles After Injection of 0.3 units/kg of HUMALOG, HUMALOG Mix50/50, HUMALOG Mix75/25, or Insulin Lispro Protamine Suspension (ILPS) in 30 Healthy Subjects.

Pharmacokinetics
Absorption — HUMALOG Mix50/50 has two phases of absorption. The early phase represents insulin lispro and its distinct characteristics of rapid onset. The late phase represents the prolonged absorption of insulin lispro protamine suspension.
In 30 healthy subjects given subcutaneous doses (0.3 unit/kg) of HUMALOG Mix50/50, the median peak serum concentration occurred at 60 minutes (range: 45 minutes to 13.5 hours) after dosing. In patients with type 1 diabetes, the median peak serum concentration occurred at 60 minutes (range: 45 minutes to 2 hours) after dosing.
Metabolism — Human metabolism studies of HUMALOG Mix50/50 have not been conducted. However, studies in animals indicate that the metabolism of HUMALOG, the rapid-acting component of HUMALOG Mix50/50, is identical to that of regular human insulin.
Elimination — Because of the absorption-rate limited kinetics of insulin mixtures, a true half-life cannot be accurately estimated from the terminal slope of the concentration versus time curve.
Specific Populations
The effects of age, race, obesity, pregnancy, smoking, or renal or hepatic impairment on the pharmacokinetics of HUMALOG Mix50/50 have not been studied.
Gender — Pharmacokinetic and pharmacodynamic comparisons between men and women administered HUMALOG Mix50/50 showed no gender differences.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Standard 2-year carcinogenicity studies in animals have not been performed with HUMALOG Mix50/50. In Fischer 344 rats, a 12-month repeat-dose toxicity study was conducted with insulin lispro (a component of HUMALOG Mix50/50) at subcutaneous doses of 20 and 200 units/kg/day (approximately 3 and 32 times the human subcutaneous dose of 1 unit insulin lispro/kg/day, based on units/body surface area). Insulin lispro did not produce important target organ toxicity including mammary tumors at any dose.
Insulin lispro was not mutagenic in the following genetic toxicity assays: bacterial mutation, unscheduled DNA synthesis, mouse lymphoma, chromosomal aberration and micronucleus assays.
Male fertility was not compromised when male rats given subcutaneous insulin lispro injections of 5 and 20 units/kg/day (0.8 and 3 times the human subcutaneous dose of 1 unit insulin lispro/kg/day, based on units/body surface area) for 6 months were mated with untreated female rats. In a combined fertility, perinatal, and postnatal study in male and female rats given 1, 5, and 20 units/kg/day subcutaneously (0.2, 0.8, and 3 times the human subcutaneous dose of 1 unit insulin lispro/kg/day, based on units/body surface area), mating and fertility were not adversely affected in either gender at any dose.
HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
HUMALOG Mix50/50 (insulin lispro protamine and insulin lispro) injectable suspension, is a white and cloudy suspension of 100 units/mL (U-100) of 50% insulin lispro protamine and 50% insulin lispro available as:
| One 10 mL multiple-dose vial | NDC 0002-7512-01 |
| Five 3 mL single-patient-use KwikPen prefilled pens | NDC 0002-8798-59 |
HUMALOG Mix50/50 KwikPen must never be shared between patients, even if the needle is changed. Patients using HUMALOG Mix50/50 vials must never share needles or syringes with another person. Always use a new disposable syringe or needle for each injection to prevent contamination.
The HUMALOG Mix50/50 KwikPen dials in 1 unit increments.
Storage and Handling
Dispense in the original sealed carton with the enclosed Instructions for Use.
Protect from direct heat and light. Do not freeze and do not use if it has been frozen. See storage table below:
a When stored at room temperature, HUMALOG Mix50/50 vial can only be used for a total of 28 days including both not in use (unopened) and in-use (opened) storage time. | |||
b When stored at room temperature, HUMALOG Mix50/50 KwikPen can only be used for a total of 10 days including both not in-use (unopened) and in-use (opened) storage time. | |||
| Not In-Use (Unopened) Refrigerated (36° to 46°F [2° to 8°C]) | Not In-Use (Unopened) Room Temperature (up to 86°F [30°C]) | In-Use (Opened) (see temperature below) | |
| 10 mL multiple-dose vial a | Until expiration date | 28 days | 28 days Refrigerated or room temperature |
| 3 mL single-patient-use KwikPen b prefilled pen | Until expiration date | 10 days | 10 days Room temperature only (Do not refrigerate) |
VIAL INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
HUMALOG ® (HU-ma-log) Mix50/50™
(insulin lispro protamine and insulin lispro)
injectable suspension, for subcutaneous use
10 mL multiple-dose vial (100 units per mL)
Read this Instructions for Use before you start taking HUMALOG Mix50/50 and each time you get a new vial. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Do not share your needles or syringes with other people, even if the needle has been changed. You may give other people a serious infection or get a serious infection from them.
Supplies needed to give your injection
- a multiple-dose HUMALOG Mix50/50 vial
- a U-100 insulin syringe and needle
- 2 alcohol swabs
- gauze
- 1 sharps container for throwing away used needles and syringes. See “ Disposing of used needles and syringes ” at the end of these instructions.
| Vial | Syringe |
 |  |
Preparing your HUMALOG Mix50/50 dose
- Wash your hands with soap and water.
- Check the HUMALOG Mix50/50 label to make sure you are taking the right type of insulin. This is especially important if you use more than 1 type of insulin.
- HUMALOG Mix50/50 is easier to mix when it is at room temperature.
- After mixing HUMALOG Mix50/50, inject your dose right away. If you wait to inject your dose, the insulin will need to be mixed again.
- Always use a new syringe and needle for each injection to help prevent infections and blocked needles. Do not reuse or share your syringes or needles with other people. You may give other people a serious infection or get a serious infection from them.
| Step 1: Gently roll the vial between the palms of your hands at least 10 times. | 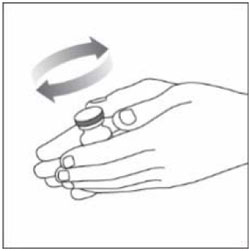 |
| Step 2: Gently move the vial up and down (invert) at least 10 times. Mixing is important to make sure you get the right dose. Humalog Mix50/50 should look white and cloudy after mixing. Do not use it if it looks clear or contains any lumps or particles. | 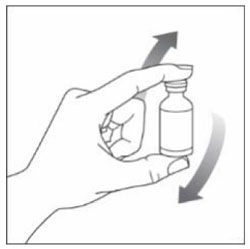 |
| Step 3: If you are using a new vial, pull off the plastic Protective Cap, but do not remove the Rubber Stopper. | 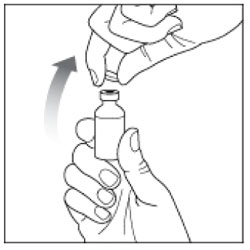 |
| Step 4: Wipe the Rubber Stopper with an alcohol swab. | 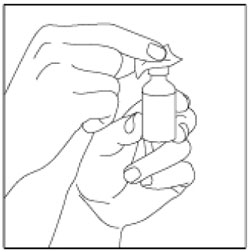 |
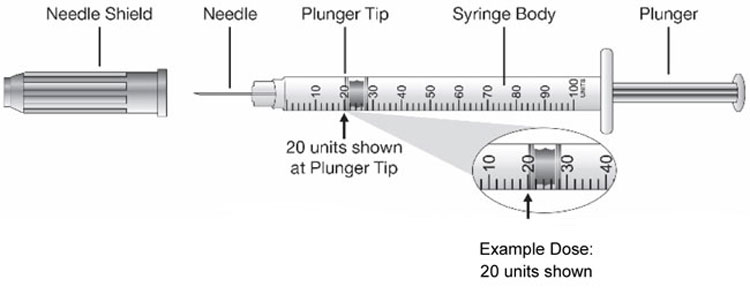
| Step 5: Remove the Needle Shield from the syringe by pulling the Needle Shield straight off. Hold the syringe with the needle pointing up. Pull down on the Plunger until the Plunger Tip reaches the line for the number of units for your prescribed dose. | 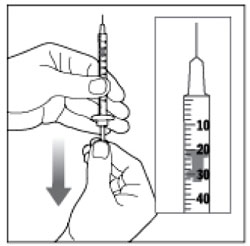 (Example Dose: 20 units shown) (Example Dose: 20 units shown) |
| Step 6: Push the needle through the Rubber Stopper of the vial. | 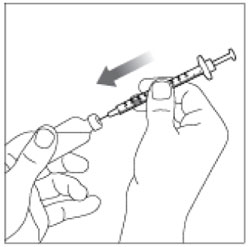 |
| Step 7: Push the Plunger all the way in. This puts air into the vial. | 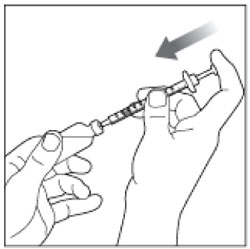 |
| Step 8: Turn the vial and syringe upside down and slowly pull the Plunger down until the Plunger Tip is a few units past the line for your prescribed dose. | 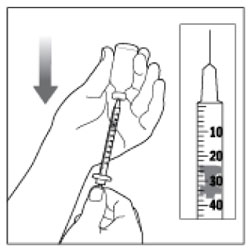 (Example Dose: 20 units Plunger Tip is shown at 24 units) (Example Dose: 20 units Plunger Tip is shown at 24 units) |
| If there are air bubbles, tap the syringe gently a few times to let any air bubbles rise to the top. | 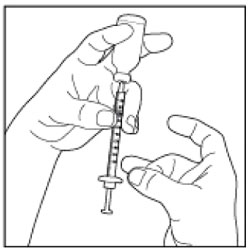 |
| Step 9: Slowly push the Plunger up until the Plunger Tip reaches the line for your prescribed dose. Check the syringe to make sure that you have the right dose. | 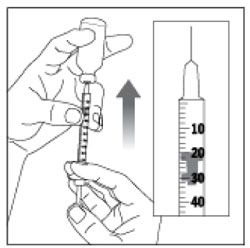 (Example Dose: 20 units shown) (Example Dose: 20 units shown) |
| Step 10: Pull the syringe out of the Rubber Stopper of the vial. | 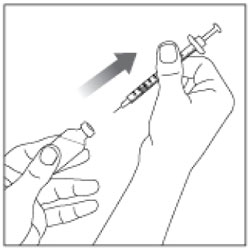 |
Giving your HUMALOG Mix50/50 injection
- Inject your insulin exactly as your healthcare provider has shown you. Your healthcare provider should tell you if you should pinch the skin before injecting.
- Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
- Do not inject where the skin has pits, is thickened, or has lumps.
- Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
| Step 11: Choose your injection site. HUMALOG Mix50/50 is injected under the skin (subcutaneously) of your stomach area (abdomen), buttocks, upper legs or upper arms. Wipe the skin with an alcohol swab. Let the injection site dry before you inject your dose. | 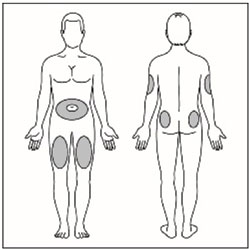 |
| Step 12: Insert the needle into your skin. | 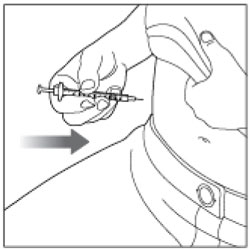 |
| Step 13: Push down on the Plunger to inject your dose. The needle should stay in your skin for at least 5 seconds to make sure you have injected all of your insulin dose. | 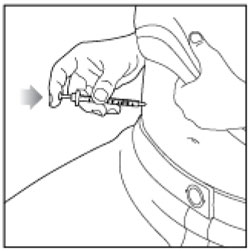 |
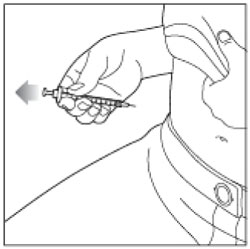
Step 14: Pull the needle out of your skin.
|  |
Disposing of used needles and syringes
- Put your used needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
How should I store HUMALOG Mix50/50?
All unopened vials:
- Store all unopened vials in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Do not freeze. Do not use if HUMALOG Mix50/50 has been frozen.
- Keep away from heat and out of direct light.
- Unopened vials can be used until the expiration date on the carton and label, if they have been stored in the refrigerator.
- Unopened vials should be thrown away after 28 days, if they are stored at room temperature.
After vials have been opened:
- Store opened vials in the refrigerator or at room temperature up to 86°F (30°C) for up to 28 days.
- Keep vials away from heat and out of direct light.
- Throw away all opened vials after 28 days of use, even if there is insulin left in the vial.
Keep HUMALOG Mix50/50 vials, syringes, needles, and all medicines out of the reach of children.
If you have any questions or problems with your HUMALOG, contact Lilly at 1-800-Lilly-Rx (1-800-545-5979) or call your healthcare provider for help. For more information on HUMALOG and insulin, go to www.humalog.com .
Manufactured by: Eli Lilly and Company Indianapolis, IN 46285, USA US License Number 1891
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 07/2023

Scan this code to launch the humalog.com website
Humalog ® and Humalog ® Mix50/50™ are registered trademarks of Eli Lilly and Company.
Copyright © 1999, 2023, Eli Lilly and Company. All rights reserved.
LOG5050VL-0003-IFU-20230721
Mechanism of Action
The primary activity of insulin including HUMALOG Mix50/50 is the regulation of glucose metabolism. Insulins lower blood glucose by stimulating peripheral glucose uptake by skeletal muscle and fat, and by inhibiting hepatic glucose production. Insulins inhibit lipolysis and proteolysis, and enhance protein synthesis.