Get your patient on Icatibant - Icatibant injection (Icatibant)
Icatibant - Icatibant injection prescribing information
INDICATIONS AND USAGE
Icatibant injection is indicated for the treatment of acute attacks of hereditary angioedema (HAE) in adults 18 years of age and older.
DOSAGE AND ADMINISTRATION
- 30 mg injected subcutaneously in the abdominal area. (2.1 )
- If response is inadequate or symptoms recur, additional injections of 30 mg may be administered at intervals of at least 6 hours. (2.1 )
- Do not administer more than 3 injections in 24 hours. (2.1 )
- Patients may self-administer upon recognition of an HAE attack. (2.2 )
Recommended Dosing
The recommended dose of Icatibant injection is 30 mg administered by subcutaneous (SC) injection in the abdominal area. Additional doses may be administered at intervals of at least 6 hours if response is inadequate or if symptoms recur. No more than 3 doses may be administered in any 24 hour period.
Administration Instructions
Icatibant injection should be inspected visually for particulate matter and discoloration prior to administration. The drug solution should be clear and colorless. Do not administer if the product contains particulates or is discolored.
Attach the provided 25 gauge needle to the syringe hub and screw on securely. Do not use a different needle. Disinfect the injection site and administer Icatibant injection by subcutaneous injection over at least 30 seconds. Discard unused portion.
Patients may self-administer Icatibant injection upon recognition of symptoms of an HAE attack after training under the guidance of a healthcare professional [see Patient Counseling Information (17 )].
DOSAGE FORMS AND STRENGTHS
Icatibant injection is supplied in a prefilled syringe delivering 30 mg icatibant. Each syringe delivers 3 mL solution with a concentration of 10 mg per mL.
USE IN SPECIFIC POPULATIONS
- Elderly patients demonstrate increased systemic exposure to icatibant. Differences in efficacy and safety between elderly and younger patients have not been identified. (8.5 )
Pregnancy
Risk Summary Available data from published literature and the pharmacovigilance database with Icatibant injection (icatibant) use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. In animal reproduction studies, icatibant, administered by the subcutaneous route during the period of organogenesis, did not cause structural abnormalities in rats or rabbits; however, premature birth and abortion were observed in rabbits at doses approximately 0.025 times the maximum recommended human dose (MRHD) and higher. Decreased embryofetal survival was observed in rabbits at a subcutaneous dose that was 13 times the MRHD. In a pre -and post-natal development study in rats, delayed parturition was observed at subcutaneous doses 0.5 times the MRHD and higher, which resulted in deaths of dams at doses 2 times the MRHD and higher. Fetal death and early pup deaths were observed with doses 2 times the MRHD ( see Data ). The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. Data Animal Data In an embryo-fetal development study with rats that received icatibant from gestation days 7 to 18, there was no evidence of any treatment-related structural abnormalities or effects on embryo-fetal survival with maternal doses up to 2.7 times the MRHD (on a mg/m 2 basis with maternal subcutaneous doses up to 25 mg/kg/day). In a fertility and early embryonic development study with rats, icatibant increased pre-implantation loss at a dose that was 7 times the MRHD (on an AUC basis at a maternal dose of 10 mg/kg/day). In an embryo-fetal development study with rabbits that received icatibant from gestation days 7 to 18, premature birth and abortion rates increased at doses approximately 0.025 times the MRHD and higher (on a mg/m 2 basis at maternal subcutaneous doses of 0.1 mg/kg and higher). Icatibant treatment resulted in dose-related decreases of total implantations and total number of live fetuses as well as dose-related increases of percent pre-implantation loss at a dose that was 13 times the MRHD (on an AUC basis with a maternal subcutaneous dose of 10 mg/kg/day). There was no evidence of any treatment-related structural abnormalities with maternal doses up to 13 times the MRHD (on an AUC basis with maternal subcutaneous doses up to 10 mg/kg/day). In a pre- and post-natal development study in the rat, dams received icatibant by the subcutaneous route at doses of 1, 3, and 10 mg/kg/day from gestation day 6 to post-partum (PPD) day 20. Delayed parturition was observed at doses 0.5 times the MRHD and higher (on an AUC basis with maternal subcutaneous doses of 1 mg/kg/day and higher), which resulted in deaths of dams at doses 2 times the MRHD and higher (on an AUC basis with maternal subcutaneous doses of 3 mg/kg/day and higher). Fetal death and increased pup deaths through PPD 4 were observed with doses 2 times the MRHD (on an AUC with a maternal subcutaneous dose of 3 mg/kg/day and higher). Impairment of pup righting reflex and decreased pup hair growth were also observed at 7 times the MRHD (on an AUC basis with a maternal dose of 10 mg/kg). Icatibant and the M2 metabolite were found in maternal milk following subcutaneous administration of icatibant. The no effect dose for F1 pups was identified at a dose 0.5 times the MRHD (on an AUC basis with a maternal subcutaneous dose of 1 mg/kg/day). A no effect dose was not identified for F 0 maternal toxicity.
Lactation
Risk Summary
There are no data on the presence of icatibant in human milk, the effects on the breastfed infant, or the effects on milk production. Icatibant and the M2 metabolite were found in rat milk following subcutaneous administration of icatibant (see Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk. However, systemic absorption of icatibant in infants is not expected after oral exposure through breast milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Icatibant injection and any potential adverse effects on the breastfed child from Icatibant injection or from the underlying maternal condition.
Data
Animal Data
Icatibant is excreted into the milk of lactating rats at concentrations that sometimes slightly exceeded those measured in the maternal plasma.
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 18 years have not been established.
Juvenile Toxicity Data
Subcutaneous daily administration of icatibant to young rats during the juvenile period of development (postnatal days 22-70) delayed the sexual maturation of male reproductive tissues (atrophy of testes and epididymides) at exposures approximating one-third or greater the MRHD on a mg/m 2 basis. Impaired fertility and reproductive performance were also observed in male rats at the end of the postnatal treatment period at exposures approximating the MRHD or greater on a mg/m 2 basis. No effects were observed in females at exposures approximating 3-fold the MRHD on a mg/m 2 basis. The observed tissue findings in males were consistent with those seen in sexually mature rats and dogs and are attributed to antagonism of the bradykinin B2 receptor and subsequent effects on gonadotropins. The observed effects may be a consequence of daily icatibant administration. Toxicity to the testis did not occur in dogs treated twice a week for 9 months [see Carcinogenesis, Mutagenesis, Impairment of Fertility (13.1 )].
Geriatric Use
Clinical studies of Icatibant injection did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Elderly patients are likely to have increased systemic exposure to Icatibant injection compared to younger (18-45 years) patients [see Clinical Pharmacology (12.3 )] . Since other reported clinical experience has not identified differences in efficacy and safety between elderly and younger patients, no dose adjustment is recommended.
Hepatic Impairment
Icatibant injection was studied in patients with mild to moderate (Child Pugh scores of 5 to 8) hepatic impairment. No change in systemic exposure is noted in these patient populations. No dose adjustment is required in patients with hepatic impairment [see Clinical Pharmacology (12.3 )].
Renal Impairment
Although a formal renal impairment study has not been conducted, 10 of 37 patients treated with Icatibant injection had hepatorenal syndrome with glomerular filtration rate (GFR) below 60 mL/min.
Icatibant injection is cleared non-renally and hence it is not expected to show any change in systemic exposure in patients with impaired renal function. No dose adjustment is required in patients with renal impairment [see Clinical Pharmacology (12.3 )].
CONTRAINDICATIONS
None.
WARNINGS AND PRECAUTIONS
- Laryngeal attacks: Following treatment of laryngeal attacks with Icatibant injection, advise patients to seek immediate medical attention. (5.1 )
Laryngeal Attacks
Given the potential for airway obstruction during acute laryngeal HAE attacks, patients should be advised to seek medical attention in an appropriate healthcare facility immediately in addition to treatment with Icatibant injection.
ADVERSE REACTIONS
The most commonly reported adverse reactions were injection site reactions, which occurred in almost all patients (97%) in clinical trials. Other common adverse reactions occurring in greater than 1% of patients included pyrexia, transaminase increase, dizziness, and rash. (6.1 ) To report SUSPECTED ADVERSE REACTIONS, contact Alembic Pharmaceuticals, Inc. at 1-866-210-9797 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Clinical Trials Experience
The safety of icatibant was evaluated in three controlled trials that included 223 patients who received Icatibant injection 30 mg (n=113), placebo (n=75), or comparator (n=38). The mean age at study entry was 38 years (range 18 to 83 years), 64% were female, and 95% were white. The data described below represent adverse reactions observed from the two placebo-controlled trials, consisting of 77 patients who received Icatibant injection at a dose of 30 mg SC, and 75 who received placebo. The most frequently reported adverse reactions (occurring in greater than 1% of patients and at a higher rate with Icatibant injection versus placebo) are shown in Table 1. Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. Table 1 Adverse reactions observed in >1% of patients with acute attacks of HAE and at a higher rate with Icatibant injection versus placebo in the placebo-controlled trials a
| ICATIBANT INJECTION (N =77) | Placebo (N = 75) | |
| System Organ Class Preferred Term | Subjects (%) | Subjects (%) |
| General disorders and administration site conditions | ||
| Injection site reaction b Pyrexia | 75 (97) 3 (4) | 25 (33) 0 |
| Investigations | ||
| Transaminase increased | 3 (4) | 0 |
| Nervous system disorders | ||
| Dizziness | 2 (3) | 1 (1) |
| a Events occurring within 14 days of study drug administration b Injection site bruising, Injection site hematoma, Injection site burning, Injection site erythema, Injection site hypoesthesia, Injection site irritation, Injection site numbness, Injection site edema, Injection site pain, Injection site pressure sensation, Injection site pruritus, Injection site swelling, Injection site urticaria, and Injection site warmth | ||
The third trial was active-controlled and was comprised of 35 patients who received Icatibant injection 30 mg and 38 patients who received the comparator. Adverse reactions for Icatibant injection were similar in nature and frequency to those reported in Table 1. In all three controlled trials, patients were eligible for treatment of subsequent attacks in an open-label extension. Patients were treated with Icatibant injection 30 mg and could receive up to 3 doses of Icatibant injection 30 mg administered at least 6 hours apart for each attack. A total of 225 patients were treated with 1,076 doses of 30 mg Icatibant injection for 987 attacks of acute HAE. Adverse reactions similar in nature and frequency were observed to those seen in the controlled phase of the trials. Other adverse reactions reported included rash, nausea, and headache in patients exposed to Icatibant injection. The safety of self-administration was evaluated in a separate, open-label trial in 56 patients with HAE. In this trial, the safety profile of Icatibant injection in patients who self-administered Icatibant injection was similar in nature and frequency to that of patients whose therapy was administered by healthcare professionals.
Immunogenicity
Across repeated treatment in the controlled trials, 4 patients tested positive for anti-icatibant antibodies. Three of these patients had subsequent tests which were negative. No hypersensitivity or anaphylactic reactions were reported with Icatibant injection. No association between anti-icatibant antibodies and efficacy was observed.
Postmarketing Experience
The following adverse reactions have been identified during post approval use of Icatibant injection: urticaria. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
DRUG INTERACTIONS
ACE Inhibitors
Icatibant injection is a bradykinin B2 receptor antagonist and thereby has the potential to have a pharmacodynamic interaction with ACE inhibitors where Icatibant injection may attenuate the antihypertensive effect of ACE inhibitors. Clinical trials to date have excluded subjects taking ACE inhibitors.
DESCRIPTION
Icatibant injection (icatibant) is a synthetic decapeptide with five non-proteinogenic amino acids. The chemical structure of icatibant acetate is presented in Figure 1.
Figure 1 Chemical Structure
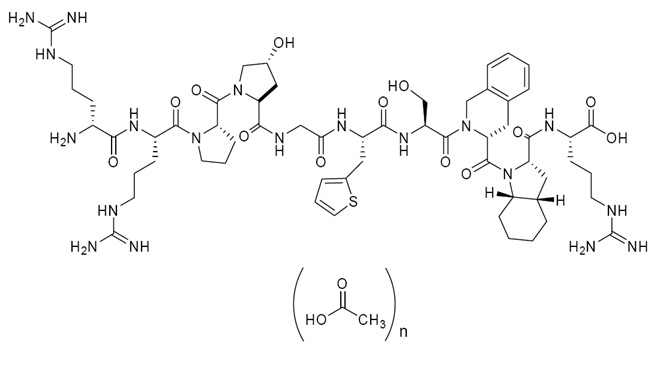
Chemical name: D-Arginyl-L-arginyl-L-prolyl-L[(4R)-4-hydroxyprolyl]-glycyl-L[3-(2-thienyl)alanyl]-Lseryl-D-(1,2,3,4-tetrahydroisoquinolin-3-ylcarbonyl)-L[(3aS,7aS) octahydroindol-2-ylcarbonyl]-L arginine, acetate salt
Icatibant injection is provided as a sterile, isotonic, and buffered solution of icatibant acetate in a single-dose, prefilled syringe for subcutaneous administration. Each mL of the solution contains 10 mg of icatibant (free base) which is equivalent to 11.38 mg of icatibant acetate. Each prefilled syringe delivers 3 mL of solution equivalent to a 30 mg icatibant dose. The solution is clear and colorless.
The solution also contains sodium chloride (isotonicity reagent), glacial acetic acid (buffering agent), sodium hydroxide (pH adjuster) and water for injection with a pH of approximately 5.5. The solution does not contain preservatives.
Pharmacological class: Icatibant is a bradykinin B2 receptor antagonist.
CLINICAL PHARMACOLOGY
Mechanism of Action
Icatibant is a competitive antagonist selective for the bradykinin B2 receptor, with an affinity similar to bradykinin. Hereditary angioedema is caused by an absence or dysfunction of C1-esterase-inhibitor, a key regulator of the Factor XII/kallikrein proteolytic cascade that leads to bradykinin production.
Bradykinin is a vasodilator which is thought to be responsible for the characteristic HAE symptoms of localized swelling, inflammation, and pain. Icatibant inhibits bradykinin from binding the B2 receptor and thereby treats the clinical symptoms of an acute, episodic attack of HAE.
Pharmacodynamics
Following bradykinin challenge, intravenous administration of Icatibant injection caused dose and time- dependent inhibition of development of bradykinin-induced hypotension, vasodilation, and reflex tachycardia in healthy young subjects. Icatibant injection intravenous doses of 0.4 and 0.8 mg/kg infused over 4 hours inhibited response to bradykinin challenge for 6 to 8 hours following completion of the infusion. Based on exposure-response analysis, a subcutaneous dose of 30 mg Icatibant injection is predicted to be effective against bradykinin challenge for at least 6 hours. The clinical significance of these findings is unknown.
The effect of Icatibant injection 30 and 90 mg following a single subcutaneous injection on QTc interval was evaluated in a randomized, placebo-, and active-controlled (moxifloxacin 400 mg) four-period crossover thorough QT study in 72 healthy subjects. In a study with demonstrated ability to detect small effects, the upper bound of the one-sided 95% confidence interval for the largest placebo adjusted, baseline-corrected QTc based on individual correction method (QTcI) was below 10 ms, the threshold for regulatory concern. The dose of 90 mg is adequate to represent the high exposure clinical scenario.
Pharmacokinetics
The pharmacokinetics of Icatibant injection has been characterized in studies using both intravenous and subcutaneous administration to healthy subjects and patients. The pharmacokinetic profile of Icatibant injection in patients with HAE is similar to that in healthy subjects.
The absolute bioavailability of Icatibant injection following a 30 mg subcutaneous dose is approximately 97%. Following subcutaneous administration of a single 30 mg dose of Icatibant injection to healthy subjects (N=96), a mean (± standard deviation) maximum plasma concentration (C max ) of 974 ± 280 ng/mL was observed after approximately 0.75 hours. The mean area under the concentration-time curve (AUC 0-∞ ) after a single 30 mg dose was 2165 ± 568 ng•hr/mL, with no evidence of accumulation of icatibant following three 30 mg doses administered 6 hours apart. Following subcutaneous administration, plasma clearance was 245 ± 58 mL/min with a mean elimination half-life of 1.4 ± 0.4 hours and volume of distribution at steady state (V ss ) of 29.0 ± 8.7 L.
Icatibant is extensively metabolized by proteolytic enzymes to inactive metabolites that are primarily excreted in the urine, with less than 10% of the dose eliminated as unchanged drug. Icatibant is not degraded by oxidative metabolic pathways, is not an inhibitor of major cytochrome P450 (CYP) isoenzymes (CYP 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1, and 3A4) and is not an inducer of CYP 1A2 and 3A4.
Special populations
Hepatic Impairment
The pharmacokinetic parameters of Icatibant injection were found to be generally comparable between healthy subjects (n=8) and mild to moderate (Child Pugh scores of 5 to 8) hepatic impaired patients (n=8) following a dose of 0.15 mg/kg/day as continuous intravenous infusion over 3 days. In a separate study, Icatibant injection clearance in subjects with a wide range of hepatic impairment (Child-Pugh scores of 7 to 15) was similar to that in healthy subjects. No dose adjustment is necessary for patients with impairment of hepatic function [see Use in Specific Populations (8.6 )].
Renal Impairment
Since renal clearance of icatibant is a minor eliminating pathway, renal impairment is not expected to affect the pharmacokinetics of Icatibant injection and hence a formal renal impairment study was not conducted for Icatibant injection. In 10 patients with hepatorenal syndrome (GFR 30-60 mL/min), clearance of Icatibant injection was not dependent on renal function and therefore, did not show any observable differences in the plasma levels of icatibant or its metabolites compared to subjects with normal renal function. No dose adjustment is necessary for patients with impairment of renal function [see Use in Specific Populations (8.7 )].
Age and Gender
Three 30 mg subcutaneous doses of Icatibant injection administered every 6 hours were studied in young (18 to 45 years of age) and elderly (over 65 years of age) healthy male and female subjects. Following single- dose administration of 30 mg subcutaneous Icatibant injection, elderly males and females showed approximately 2-fold higher AUC compared to young males and females, respectively. However, only minor differences (~12-14%) between C max of gender–matched elderly and young subjects were observed.
Older subjects tend to exhibit lower clearance compared to younger subjects and therefore higher systemic exposure. Gender effect on Icatibant injection pharmacokinetics was also observed in addition to age effect. Clearance of Icatibant injection is significantly correlated with bodyweight with lower clearance values noted for lower bodyweights. Hence, females with typically lower body weights compared to males exhibit lower clearance values, resulting in approximately 2-fold higher systemic exposure (both AUC and C max ) compared to males. Differences in efficacy and safety between elderly and younger patients and male and female patients have not been identified. Dose adjustment based on age and gender is not warranted.
Drug Interactions
Formal drug-drug interaction studies were not conducted with Icatibant injection. Icatibant metabolism is not mediated by CYP450 enzymes. In vitro study did not show any significant inhibition and/or induction of drug metabolizing CYP450 enzymes; therefore, metabolic drug interactions between Icatibant injection and CYP450 substrates, inhibitors and inducers are not expected.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year studies were conducted in CD1 mice and Wistar rats to assess the carcinogenic potential of Icatibant injection. No evidence of tumorigenicity was observed in mice and rats at icatibant subcutaneous doses up to 15 mg/kg/day (twice per week) and 6 mg/kg/day (daily), respectively (approximately 10-fold and 6 -fold greater than the MRHD on an AUC basis, respectively).
Icatibant tested negative for genotoxicity in the in vitro Ames bacterial reverse mutation test, in vitro Chinese hamster bone marrow chromosome aberration assay, and in vivo mouse micronucleus test.
Daily subcutaneous administration of icatibant to rats and dogs caused ovarian, uterine, and testicular atrophy/degeneration and adverse effects on the mammary and prostate glands. In rats, testicular atrophy, reduced prostate gland secretion, decreased testosterone levels and degenerate corpora lutea occurred at doses greater than or equal to 3 mg/kg (approximately 5-fold greater than the MRHD in males and 2-fold greater than the MRHD in females on an AUC basis) and a decrease in developing ovarian follicles, mammary gland masculinization, and uterine atrophy occurred at doses greater than or equal to 10 mg/kg (approximately 6-fold greater than MRHD in females on an AUC basis). In dogs, reduced sperm counts and uterine atrophy occurred at doses greater than or equal to 1 mg/kg (approximately 2-fold greater than the MRHD on an AUC basis). Atrophy of the testes and prostate with decreased testosterone levels, decreased ovary size and decreased number of developing follicles occurred at a dose of 10 mg/kg (approximately 30-fold greater than the MRHD in males and 15-fold greater than at the MRHD in females on an AUC basis).
In contrast to the effects of daily icatibant administration, toxicity to the ovary, uterus, testis, mammary gland, and prostate did not occur in dogs treated twice a week for 9 months. AUC exposures from a dose of 3 mg/kg in these dogs were 5- and 3-fold the MRHD exposures in men and women, respectively.
Sperm counts and testosterone remained unaffected over the course of the study in male dogs dosed twice a week.
Reproduction studies in male mice and rats with daily administration of icatibant found no effects on fertility or reproductive performance with intravenous doses up to 81 mg/kg (approximately 5-fold greater than the MRHD on a mg/m 2 basis) or subcutaneous doses up to 10 mg/kg (approximately 11-fold greater than the MRHD on an AUC basis), respectively.
Animal Toxicology and/or Pharmacology
The B2 receptor has been implicated in the cardioprotective effects of bradykinin and antagonism of this receptor could potentially have negative cardiovascular effects during reperfusion after acute ischemia. Icatibant decreased coronary blood flow in the isolated guinea pig heart and aggravated the duration of post-ischemic reperfusion arrhythmias in the isolated rat heart. Intracoronary infusion of icatibant in an anesthetized myocardial infarction dog model increased mortality rate 2-fold over saline ischemia. There is limited human experience in acute ischemia. Icatibant injection should be used during acute coronary ischemia, unstable angina pectoris, or in the weeks following a stroke only if the benefit exceeds the theoretical risk to the patient.
CLINICAL STUDIES
The efficacy and safety of Icatibant injection for the treatment of acute attacks of HAE in adults were studied in three controlled clinical trials. Among the 223 patients in these studies, the mean age was 38 years, 64% were female, and 95% were white. Approximately 57% of patients reported use of attenuated androgens, antifibrinolytic agents, or C1 inhibitors. Response to therapy was primarily assessed using visual analog scores on a 100 mm scale and patient- and physician-reported symptom scores for abdominal and cutaneous pain and swelling.
Trial 1 was a randomized, placebo-controlled, double-blind, parallel-group study of 98 adult patients with a median age of 36 years. Patients who had developed moderate to severe cutaneous or abdominal or mild to moderate laryngeal attacks of HAE were randomized to receive either Icatibant injection 30 mg or placebo by subcutaneous injection. Patients with severe laryngeal attacks of HAE received open-label Icatibant injection 30 mg. The primary endpoint was assessed using a 3-item composite visual analog score (VAS), comprised of averaged assessments of skin swelling, skin pain, and abdominal pain. Response was defined as at least a 50% reduction from the pretreatment composite 3-item VAS score (Figure 2). The median time to 50% reduction in symptoms for patients with cutaneous or abdominal attacks treated with Icatibant injection (n=43) compared to placebo (n=45) was 2.0 hours [95% CI 1.5, 3.0] versus 19.8 hours [95% CI 6.1, 26.3], respectively (p<0.001).
Figure 2 Time to 50% reduction from baseline in 3-item VAS score. 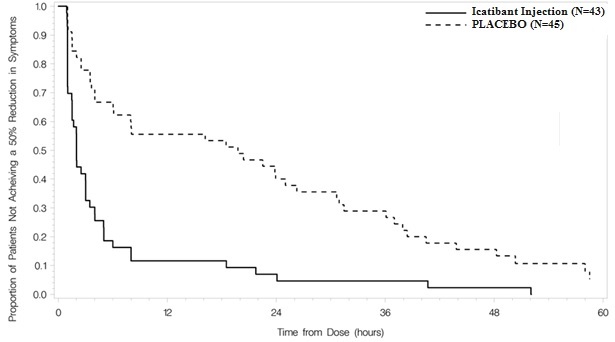
Other evaluated endpoints included time to almost complete symptom relief (VAS<10 mm) and rescue medication use. In Trial 1, the median times to almost complete symptom relief were 8.0 versus 36.0 hours for Icatibant injection and placebo, respectively. In terms of rescue medication use, 3/43 (7%) patients treated with Icatibant injection used additional rescue medication in comparison to 18/45 (40%) patients treated with placebo.
In a second placebo-controlled trial and an active-controlled trial, a total of 26 and 35 patients, respectively, received Icatibant injection 30 mg for the treatment of an acute HAE attack. Across the three trials, Icatibant injection had a median time to 50% reduction from baseline symptoms ranging from 2.0 to 2.3 hours.
Recurrent attacks
In all three controlled trials, patients were eligible for treatment of subsequent attacks in an open-label extension. Patients were treated with Icatibant injection 30 mg and could receive up to 3 doses of Icatibant injection 30 mg administered at least 6 hours apart for each attack. A total of 225 patients were treated with 1,076 doses of 30 mg Icatibant injection for 987 attacks of acute HAE in these trials. In an assessment of the first 5 Icatibant injection-treated attacks (621 doses for 582 attacks), the median times to a 50% reduction from the pretreatment composite 3-item VAS score were similar across attacks (2.0, 2.0, 2.4, 2.0, 1.5 hours). The majority (93%) of these attacks of HAE were treated with a single dose of Icatibant injection.
Laryngeal attacks
A total of 60 patients with laryngeal attacks were treated with Icatibant injection in the controlled trials. Efficacy results were similar to those observed for non-laryngeal (cutaneous and abdominal) sites of attack.
Self-administration
Self-administration of Icatibant injection by 56 patients was assessed in an open label trial. Patients who administered Icatibant injection during an acute attack of HAE had a median time to 50% reduction from the pretreatment composite 3-item VAS score of 2.6 hours.
HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Icatibant injection is supplied as a sterile, clear colorless solution in single-dose, prefilled syringe for subcutaneous administration. Each syringe delivers 3 mL of a sterile solution of icatibant 30 mg (as icatibant acetate). Each glass syringe has a bromobutyl plunger stopper, which is not made of latex natural rubber.
Icatibant injection is available in cartons containing one single-dose, prefilled syringe and one 25 G Luer lock needle. NDC 62332-654-03.
Icatibant injection is also available in a pack containing 3 cartons; each carton contains one single-dose, prefilled syringe and one 25 G Luer lock needle. NDC 62332-654-09.
Storage and Handling
Keep out of the reach of children. Store between 2 to 25° C (36 to 77° F).
Do not freeze.
Store in carton until time of administration.
Mechanism of Action
Icatibant is a competitive antagonist selective for the bradykinin B2 receptor, with an affinity similar to bradykinin. Hereditary angioedema is caused by an absence or dysfunction of C1-esterase-inhibitor, a key regulator of the Factor XII/kallikrein proteolytic cascade that leads to bradykinin production.
Bradykinin is a vasodilator which is thought to be responsible for the characteristic HAE symptoms of localized swelling, inflammation, and pain. Icatibant inhibits bradykinin from binding the B2 receptor and thereby treats the clinical symptoms of an acute, episodic attack of HAE.