Igalmi
(Dexmedetomidine)Dosage & Administration
By using PrescriberAI, you agree to the AI Terms of Use.
Igalmi Prescribing Information
IGALMI is indicated for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder in adults.
- IGALMI should be administered under the supervision of a healthcare provider. A healthcare provider should monitor vital signs and alertness after IGALMI administration to prevent falls and syncope. ()
2.1 Important Recommendations Prior to Initiating IGALMI and During TherapyIGALMI should be administered under the supervision of a healthcare provider. A healthcare provider should monitor vital signs and alertness after IGALMI administration to prevent falls and syncope
[see Warnings and Precautions (5.5)].IGALMI is for sublingual or buccal administration. Do not chew or swallow IGALMI. Do not eat or drink for at least 15 minutes after sublingual administration, or at least one hour after buccal administration.
- Administer sublingually or buccally. Do not chew or swallow. ()
2.1 Important Recommendations Prior to Initiating IGALMI and During TherapyIGALMI should be administered under the supervision of a healthcare provider. A healthcare provider should monitor vital signs and alertness after IGALMI administration to prevent falls and syncope
[see Warnings and Precautions (5.5)].IGALMI is for sublingual or buccal administration. Do not chew or swallow IGALMI. Do not eat or drink for at least 15 minutes after sublingual administration, or at least one hour after buccal administration.
- Recommended dosage ():
2.2 Recommended DosageTable 1 includes dosage recommendations for IGALMI based on agitation severity for adults, patients with hepatic impairment, and geriatric patients. Lower dosages are recommended for patients with hepatic impairment and geriatric patients
[see Warnings and Precautions (5.1)and Use in Specific Populations (8.5, 8.6)].If agitation persists after the initial dose, up to two additional doses may be administered at least two hours apart. The dosage recommendations for additional doses vary depending upon the patient population and agitation severity (see Table 1). Assess vital signs including orthostatic measurements prior to the administration of any subsequent doses.
Due to risk of hypotension, additional half-doses are not recommended in patients with systolic blood pressure (SBP) less than 90 mmHg, diastolic blood pressure (DBP) less than 60 mmHg, heart rate (HR) less than 60 beats per minute, or postural decrease in SBP ≥ 20 mmHg or in DBP ≥ 10 mmHg.
Table 1: Dosage Recommendations for IGALMI in Adults, Adult Patients with Hepatic Impairment, and Geriatric Patients with Agitation Associated with Schizophrenia or Bipolar I or II Disorder Patient Population Agitation Severity Initial DoseIGALMI 120 mcg and 180 mcg dosage strengths may be cut in half to obtain the 60 mcg and 90 mcg doses, respectively [see Dosage and Administration (2.3)].Optional 2nd/3rdDoses Maximum Recommended Total Daily Dosage Adults Mild or Moderate 120 mcg 60 mcg 240 mcg Severe 180 mcg 90 mcg 360 mcg Patients with Mild or Moderate Hepatic ImpairmentHepatic impairment: Mild (Child-Pugh Class A); Moderate (Child-Pugh Class B); Severe (Child-Pugh Class C) Mild or Moderate 90 mcg 60 mcg 210 mcg Severe 120 mcg 60 mcg 240 mcg Patients with Severe Hepatic Impairment Mild or Moderate 60 mcg 60 mcg 180 mcg Severe 90 mcg 60 mcg 210 mcg Geriatric Patients
(≥ 65 years old)Mild, Moderate, or Severe 120 mcg 60 mcg 240 mcg Patient Population Agitation Severity Initial DoseSee Full Prescribing Information for recommendations on administering up to two additional doses and maximum recommended dosages. Adults Mild or Moderate 120 mcg Severe 180 mcg Mild or Moderate Hepatic Impairment Mild or Moderate 90 mcg Severe 120 mcg Severe Hepatic Impairment Mild or Moderate 60 mcg Severe 90 mcg Geriatric Patients
(≥ 65 years old)Mild, Moderate, or Severe 120 mcg - IGALMI 120 mcg and 180 mcg dosage strengths may be cut in half to obtain the 60 mcg and 90 mcg doses, respectively. See Full Prescribing Information for preparation and administration instructions. ()
2.3 Preparation and Administration InstructionsKeep IGALMI in the foil pouch until ready to administer. IGALMI should be immediately administered once the pouch is opened and the dose prepared.
Prepare and administer IGALMI under the supervision of a healthcare provider as follows:
Healthcare Professional: Prepare IGALMI Dose for Patient1Open the sealed foil pouch by tearing straight across at the notch. 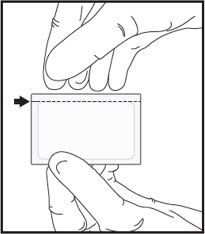
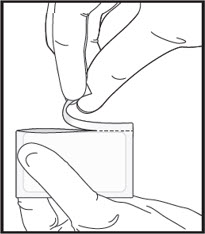
- Perform Steps 2a, 2b, 2c and 2d only if a 60 mcg or 90 mcg dose (half of a film) is needed, then proceed to Step 3.
- If administering a full dose (1 film), proceed directly to Step 3.
2aRemove the film from the pouch with clean dry hands. 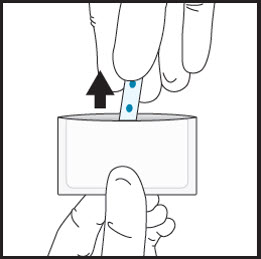 2b
2bCut the film in half between the dots with clean, dry scissors. 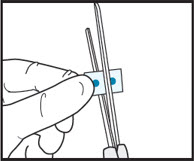
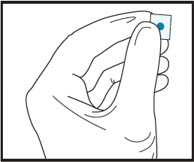 2cDiscard unused half in waste container.2d
2cDiscard unused half in waste container.2dPlace the half film for administration to the patient back into the pouch. 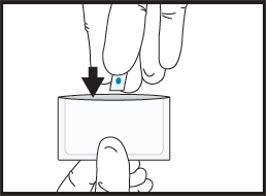 3
3Immediately give the pouch to the patient. 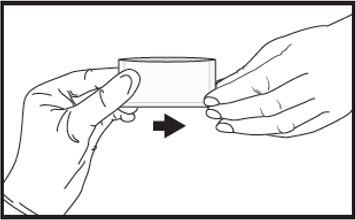 4
4Instruct patient to remove the film from the pouch with clean dry hands. 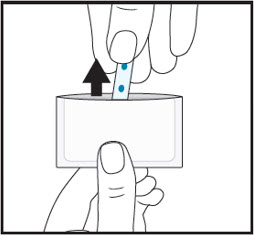 5For sublingual administration:Instruct patient to place film under the tongue. The film will stick in place.Note:Patient may not eat or drink for 15 minutes after sublingual administration.
5For sublingual administration:Instruct patient to place film under the tongue. The film will stick in place.Note:Patient may not eat or drink for 15 minutes after sublingual administration.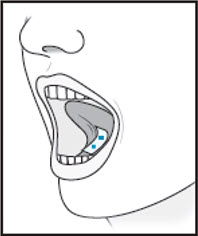 For buccal administration:Instruct patient to place film behind lower lip. The film will stick in place.Note:Patient may not eat or drink for one hour after buccal administration.
For buccal administration:Instruct patient to place film behind lower lip. The film will stick in place.Note:Patient may not eat or drink for one hour after buccal administration.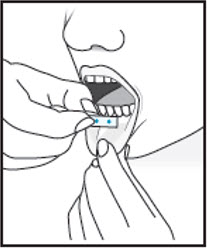 6
6Instruct patient to: - Close their mouth.
- Allow the film to dissolve.
- Do not chew or swallow the film.

Figure 
Figure 
Figure 
Figure 
Figure 
Figure 
Figure 
Figure 
Figure 
Figure
IGALMI is a blue rectangular sublingual film containing on its surface two darker blue spots in dose strengths of 120 mcg and 180 mcg.
There are no available data on IGALMI use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal effects. Available data from published randomized controlled trials and case reports over several decades of use with intravenously administered dexmedetomidine during pregnancy have not identified a drug-associated risk of major birth defects or miscarriage; however, the reported exposures occurred after the first trimester. Most of the available data are based on studies with exposures that occurred at the time of cesarean-section delivery, and these studies have not identified an adverse effect on maternal outcomes or infant Apgar scores. Available data indicate that dexmedetomidine crosses the placenta.
In animal reproductive studies fetal toxicity occurred in the presence of maternal toxicity with subcutaneous administration of dexmedetomidine to pregnant rats during organogenesis at doses 5 times the maximum recommended human dose [MRHD] of 360 mcg/day based on mg/m2 body surface area. Adverse developmental effects, including early implantation loss and decreased viability of second generation offspring, occurred when pregnant rats were subcutaneously administered doses less than or equal to the MRHD based on mg/m2 from late pregnancy through lactation and weaning
Increased post-implantation losses and reduced live pups in the presence of maternal toxicity (decreased body weight) occurred in a rat embryo-fetal development study in which pregnant dams were administered subcutaneous doses of dexmedetomidine of 200 mcg/kg/day (equivalent to 5 times the MRHD of 360 mcg/day based on mg/m2) during the period of organogenesis (Gestation Day (GD) 5 to 16). No embryo-fetal toxicity was observed at 20 mcg/kg/day (less than the MRHD of 360 mcg/day based on mg/m2). No malformations were reported at any dose level.
No malformation or embryo-fetal toxicity were observed in a rabbit embryo-fetal developmental study in which pregnant dams were administered dexmedetomidine intravenously at doses up to 96 mcg/kg/day (equivalent to 5 times the MRHD of 360 mcg/day based on mg/m2) during the period of organogenesis (GD 6 to 18).
Reduced pup and adult offspring weights and grip strength were reported in a rat developmental toxicology study in which pregnant females were administered dexmedetomidine subcutaneously at 8 mcg/kg/day (less than the MRHD of 360 mcg/day based on mg/m2) during late pregnancy through lactation and weaning (GD 16 to postnatal day [PND] 25). Decreased viability of second generation offspring and an increase in early implantation loss along with delayed motor development occurred at 32 mcg/kg/day (equivalent to the MRHD of 360 mcg/day based on mg/m2) when first generation offspring were mated. This study limited dosing to hard palate closure (GD 15-18) through weaning instead of standard dosing from implantation (GD 6-7) to weaning (PND 21).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
None.
- Hypotension, Orthostatic Hypotension, and Bradycardia:Avoid use of IGALMI in patients with hypotension, orthostatic hypotension, advanced heart block, severe ventricular dysfunction, or history of syncope. Ensure that patients are alert and not experiencing orthostatic or symptomatic hypotension prior to resuming ambulation. ()
5.1 Hypotension, Orthostatic Hypotension, and BradycardiaIGALMI causes dose-dependent hypotension, orthostatic hypotension, and bradycardia
.In clinical studies, 18%, 16%, and 9% of patients treated with 180 mcg of IGALMI, 120 mcg of IGALMI, and placebo, respectively, experienced orthostatic hypotension (defined as SBP decrease ≥ 20 mmHg or DBP decrease ≥ 10 mmHg after 1, 3, or 5 minutes of standing) at 2 hours post-dose. In those studies, 7%, 6%, and 1% of patients treated with 180 mcg of IGALMI, 120 mcg of IGALMI, and placebo, respectively, experienced HR ≤ 50 beats per minute within 2 hours of dosing[see Adverse Reactions (6.1)].In clinical studies with IGALMI, patients were excluded if they had treatment with alpha-1 noradrenergic blockers, benzodiazepines, other hypnotics or antipsychotic drugs four hours prior to study drug administration; had a history of syncope or syncopal attacks; SBP < 110 mmHg; DBP < 70 mmHg; HR < 55 beats per minute; or had evidence of hypovolemia or orthostatic hypotension.Reports of hypotension and bradycardia, including some resulting in fatalities, have been associated with the use of another dexmedetomidine product given intravenously (IGALMI is for sublingual or buccal use and is not approved for intravenous use). Clinically significant episodes of bradycardia and sinus arrest have been reported after administration of this other dexmedetomidine product to young, healthy adult volunteers with high vagal tone and when this product was given by rapid intravenous or bolus administration.
Because IGALMI decreases sympathetic nervous system activity, hypotension and/or bradycardia may be more pronounced in patients with hypovolemia, diabetes mellitus, or chronic hypertension, and in geriatric patients
[see Dosage and Administration (2.2)and Use in Specific Populations (8.5)].Avoid use of IGALMI in patients with hypotension, orthostatic hypotension, advanced heart block, severe ventricular dysfunction, or history of syncope. After IGALMI administration, patients should be adequately hydrated and should sit or lie down until vital signs are within normal range. If a patient is unable to remain seated or lying down, precautions should be taken to reduce the risk of falls. Ensure that a patient is alert and not experiencing orthostatic hypotension or symptomatic hypotension prior to allowing them to resume ambulation
[see Dosage and Administration (2.1)]. - QT Interval Prolongation:IGALMI prolongs the QT interval; avoid use in patients with risk factors for prolonged QT interval. ()
5.2 QT Interval ProlongationIGALMI prolongs the QT interval. Avoid use of IGALMI in patients at risk of torsades de pointes or sudden death including those with known QT prolongation, a history of other arrhythmias, symptomatic bradycardia, hypokalemia or hypomagnesemia, and in patients receiving other drugs known to prolong the QT interval
[see Drug Interactions (7.1)and Clinical Pharmacology (12.2)]. - Somnolence:Patients should not perform activities requiring mental alertness, such as operating a motor vehicle or operating hazardous machinery for at least eight hours after taking IGALMI. ()
5.3 SomnolenceIGALMI can cause somnolence. In placebo-controlled clinical studies in adults with agitation associated with schizophrenia or bipolar I or II disorder, somnolence (including fatigue and sluggishness) was reported in 23% and 22% of patients treated with IGALMI 180 mcg and 120 mcg, respectively, compared to 6% of placebo-treated patients. Patients should not perform activities requiring mental alertness, such as operating a motor vehicle or operating hazardous machinery, for at least eight hours after taking IGALMI
[see Adverse Reactions (6.1)].