Get your patient on Iluvien (Fluocinolone Acetonide)
Iluvien prior authorization resources
Most recent state uniform prior authorization forms
Brand Resources
Iluvien patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
General Dosing Information
For ophthalmic intravitreal injection.
The initial prescription and renewal of the medication order of ILUVIEN should be made by a physician only after examination of the patient with the aid of magnification, such as slit lamp biomicroscopy, and, where appropriate, fluorescein staining.
Administration
The intravitreal injection procedure should be carried out under aseptic conditions, which include use of sterile gloves, a sterile drape, a sterile caliper, and a sterile eyelid speculum (or equivalent). Adequate anesthesia and a broad-spectrum microbicide should be given prior to the injection.
The injection procedure for ILUVIEN is as follows:
- The exterior of the tray should notbe considered sterile. An assistant (non-sterile) should remove the tray from the carton and examine the tray and lid for damage. If damaged, do not use unit.
If acceptable, the assistant should peel the lid from the tray without touching the interior surface. - Visually check through the viewing window of the preloaded applicator to ensure that there is a drug implant inside.
- Remove the applicator from the tray with sterile gloved hands touching only the sterile interior tray surface and applicator.
Prior to injection, the applicator tip must be kept above the horizontal plane to ensure that the implant is properly positioned within the applicator. - To reduce the amount of air administered with the implant, the administration procedure requires two steps. Before inserting the needle into the eye, remove the protective cap then gently push the applicator button down and slide it to the first stop (at the curved black marks alongside the button track). At the first stop, release the button and it should move to the UP position. If the button does not rise to the UP position, do not proceed with this unit.
- Optimal placement of the implant is inferior to the optic disc and posterior to the equator of the eye. Measure 4 millimeters inferotemporal from the limbus with the aid of calipers for point of entry into the sclera.
- Inspect the tip of the needle to ensure it is not bent.
- Gently displace the conjunctiva so that after withdrawing the needle, the conjunctival and scleral needle entry sites will not align. Care should be taken to avoid contact between the needle and the lid margin or lashes. Insert the needle through the conjunctiva and sclera. To release the implant, while the button is in the UP position, advance the button by sliding it forward to the end of the button track and remove the needle. Note: Ensure that the button reaches the end of the track before removing the needle.
- Remove the lid speculum and perform indirect ophthalmoscopy to verify placement of the implant, adequate central retinal artery perfusion and absence of any other complications.
Following the injection, patients should be monitored for change in intraocular pressure and for endophthalmitis. Monitoring may consist of a check for perfusion of the optic nerve head immediately after the injection, tonometry within 30 minutes following the injection, and biomicroscopy between two and seven days following the injection. Patients should be instructed to report without delay any symptoms suggestive of endophthalmitis.

By using PrescriberAI, you agree to the AI Terms of Use.
Iluvien prescribing information
Indications and Usage, Chronic Non-Infectious Uveitis Affecting the Posterior Segment (1.2) 03/2025
INDICATIONS AND USAGE
ILUVIEN is a corticosteroid indicated for:
Diabetic Macular Edema
ILUVIEN ® is indicated for the treatment of diabetic macular edema (DME) in patients who have been previously treated with a course of corticosteroids and did not have a clinically significant rise in intraocular pressure.
Chronic Non-Infectious Uveitis Affecting the Posterior Segment
ILUVIEN ® is indicated for the treatment of chronic non-infectious uveitis affecting the posterior segment of the eye.
DOSAGE AND ADMINISTRATION
General Dosing Information
For ophthalmic intravitreal injection.
The initial prescription and renewal of the medication order of ILUVIEN should be made by a physician only after examination of the patient with the aid of magnification, such as slit lamp biomicroscopy, and, where appropriate, fluorescein staining.
Administration
The intravitreal injection procedure should be carried out under aseptic conditions, which include use of sterile gloves, a sterile drape, a sterile caliper, and a sterile eyelid speculum (or equivalent). Adequate anesthesia and a broad-spectrum microbicide should be given prior to the injection.
The injection procedure for ILUVIEN is as follows:
- The exterior of the tray should notbe considered sterile. An assistant (non-sterile) should remove the tray from the carton and examine the tray and lid for damage. If damaged, do not use unit. If acceptable, the assistant should peel the lid from the tray without touching the interior surface.
- Visually check through the viewing window of the preloaded applicator to ensure that there is a drug implant inside.
- Remove the applicator from the tray with sterile gloved hands touching only the sterile interior tray surface and applicator. Prior to injection, the applicator tip must be kept above the horizontal plane to ensure that the implant is properly positioned within the applicator.
- To reduce the amount of air administered with the implant, the administration procedure requires two steps. Before inserting the needle into the eye, remove the protective cap then gently push the applicator button down and slide it to the first stop (at the curved black marks alongside the button track). At the first stop, release the button and it should move to the UP position. If the button does not rise to the UP position, do not proceed with this unit.
- Optimal placement of the implant is inferior to the optic disc and posterior to the equator of the eye. Measure 4 millimeters inferotemporal from the limbus with the aid of calipers for point of entry into the sclera.
- Inspect the tip of the needle to ensure it is not bent.
- Gently displace the conjunctiva so that after withdrawing the needle, the conjunctival and scleral needle entry sites will not align. Care should be taken to avoid contact between the needle and the lid margin or lashes. Insert the needle through the conjunctiva and sclera. To release the implant, while the button is in the UP position, advance the button by sliding it forward to the end of the button track and remove the needle. Note: Ensure that the button reaches the end of the track before removing the needle.
- Remove the lid speculum and perform indirect ophthalmoscopy to verify placement of the implant, adequate central retinal artery perfusion and absence of any other complications.
Following the injection, patients should be monitored for change in intraocular pressure and for endophthalmitis. Monitoring may consist of a check for perfusion of the optic nerve head immediately after the injection, tonometry within 30 minutes following the injection, and biomicroscopy between two and seven days following the injection. Patients should be instructed to report without delay any symptoms suggestive of endophthalmitis.
DOSAGE FORMS AND STRENGTHS
ILUVIEN is a non-bioerodable intravitreal implant in a drug delivery system containing 0.19 mg fluocinolone acetonide, designed to release fluocinolone acetonide at an initial rate of 0.25 mcg/day and lasting 36 months.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
There are no adequate and well-controlled studies of ILUVIEN use in pregnant women to inform a drug-associated risk. Animal reproduction studies have not been conducted with fluocinolone acetonide. Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. It is not known whether ILUVIEN can cause fetal harm when administered to a pregnant women or affect reproduction capacity. ILUVIEN should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the United States general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Lactation
Risk Summary
Systemically administered corticosteroids that are present in human milk can suppress growth and interfere with endogenous corticosteroid production. Clinical or nonclinical lactation studies have not been conducted with ILUVIEN. The systemic concentration of fluocinolone acetonide following intravitreal treatment with ILUVIEN is low [see Clinical Pharmacology (12.3) ]. It is not known whether intravitreal treatment with ILUVIEN could result in sufficient systemic absorption to produce detectable quantities in human milk, or affect breastfed infants or milk production. Exercise caution when ILUVIEN is administered to a nursing woman. The developmental and health benefits of breastfeeding should be considered, along with the mother’s clinical need for ILUVIEN and any potential adverse effects on the breastfed infant from ILUVIEN or from the underlying maternal condition.
Pediatric Use
The safety and effectiveness of ILUVIEN have not been established in pediatric patients.
Geriatric Use
No overall differences in safety or effectiveness have been observed between elderly and younger patients.
CONTRAINDICATIONS
Ocular or Periocular Infections
ILUVIEN is contraindicated in patients with active or suspected ocular or periocular infections including most viral disease of the cornea and conjunctiva including active epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, varicella, mycobacterial infections and fungal diseases.
Glaucoma
ILUVIEN is contraindicated in patients with glaucoma, who have cup to disc ratios of greater than 0.8.
Hypersensitivity
ILUVIEN is contraindicated in patients with known hypersensitivity to any components of this product.
WARNINGS AND PRECAUTIONS
- Intravitreal injections have been associated with endophthalmitis, eye inflammation, increased intraocular pressure, and retinal detachments. Patients should be monitored following the injection. (5.1)
- Intraocular Pressure (IOP) Increase : Prolonged use of corticosteroids may result in glaucoma with damage to the optic nerve, defects in visual acuity, and fields of vision. (5.2)
- Cataracts : Use of corticosteroids may result in posterior subcapsular cataract formation. (5.3)
- Delayed Healing : The use of corticosteroids after cataract surgery may delay healing and increase the incidence of bleb formation. (5.4)
- Corneal and Scleral Melting : In those diseases causing thinning of the cornea or sclera, ophthalmic corticosteroids may lead to perforation of the globe. (5.5)
- Bacterial Infections : Prolonged use of corticosteroids may suppress the host response and thus increase the hazard of secondary ocular infections. In acute purulent conditions, steroids may mask infection or enhance existing infection. If signs and symptoms fail to improve after 2 days, the patient should be re-evaluated. (5.6)
- Viral Infections : Employment of a corticosteroid medication in the treatment of patients with a history of herpes simplex requires great caution. Use of ocular steroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes simplex). (5.7)
- Fungal Infections : Fungal infections of the cornea are particularly prone to develop coincidentally with long-term local corticosteroid application. Fungus invasion must be considered in any persistent corneal ulceration where a steroid has been used or is in use. (5.8)
- Implant Migration : The implant may migrate into the anterior chamber if the posterior lens capsule is not intact. (5.9)
Intravitreal Injection-related Effects
Intravitreal injections, including those with ILUVIEN, have been associated with endophthalmitis, eye inflammation, increased or decreased intraocular pressure, and choroidal or retinal detachments. For patients with non-infectious uveitis affecting the posterior segment, hypotony has been observed within 24 hours of injection and has resolved within 2 weeks. Patients should be monitored following the intravitreal injection [see Patient Counseling Information (17) ] . Patients may experience temporary blurred vision after injection of the implant.
Intraocular Pressure (IOP) Increase
Prolonged use of corticosteroids may result in the development of glaucoma with damage to the optic nerve, defects in visual acuity and fields of vision. Steroids should be used with caution in the presence of glaucoma. Intraocular pressure should be routinely monitored during the course of the treatment.
Cataracts
The use of corticosteroids may result in posterior subcapsular cataract formation.
Delayed Corneal Wound Healing
The use of corticosteroids after cataract surgery may delay healing and increase the incidence of bleb formation.
Corneal and Scleral Melting
Various ocular diseases and long-term use of topical corticosteroids have been known to cause corneal and scleral thinning. Use of ophthalmic corticosteroids in the presence of thin corneal or scleral tissue may lead to perforation of the globe.
Bacterial Infections
Prolonged use of corticosteroids may suppress the host immune response and thus increase the hazard of secondary ocular infections. Acute purulent or parasitic infections of the eye may be masked or activity enhanced by the presence of corticosteroid medication. If signs and symptoms fail to improve after 2 days, the patient should be reevaluated.
Viral Infections
Use of ocular corticosteroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes simplex). Employment of a corticosteroid medication in the treatment of patients with a history of herpes simplex requires great caution; frequent slit lamp microscopy is recommended.
Fungal Infections
Fungal infections of the cornea are particularly prone to develop coincidentally with long-term local corticosteroid application. Fungus invasion should be suspected in any persistent corneal ulceration where a corticosteroid has been used or is in use. Fungal cultures should be taken when appropriate.
Risk of Implant Migration
Patients in whom the posterior capsule of the lens is absent or has a tear are at risk of implant migration into the anterior chamber.
ADVERSE REACTIONS
The most common adverse reactions reported are cataract development and increases in intraocular pressure. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Alimera Sciences, Inc. at 1-844-445-8843 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse reactions associated with ophthalmic steroids including ILUVIEN include cataract formation and subsequent cataract surgery, elevated intraocular pressure, which may be associated with optic nerve damage, visual acuity and field defects, secondary ocular infection from pathogens including herpes simplex, and perforation of the globe where there is thinning of the cornea or sclera.
Diabetic Macular Edema
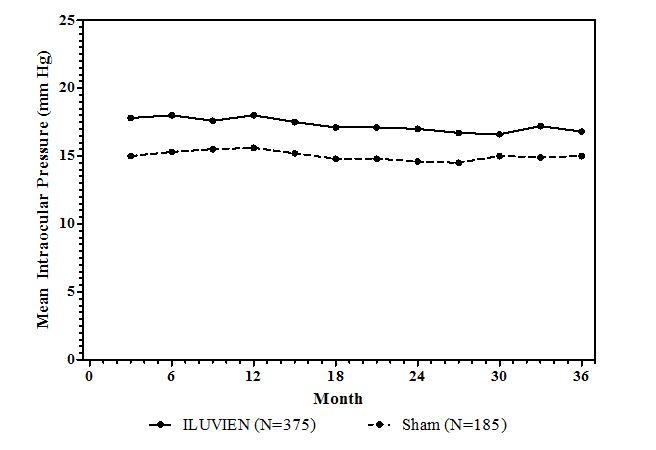
ILUVIEN was studied in two multicenter, randomized, sham-controlled, double-masked trials in which patients with diabetic macular edema (DME) were treated with either ILUVIEN (n=375) or sham (n=185).
Table 1 summarizes safety data available when the last subject completed the last 36 month follow up visit for the two primary ILUVIEN trials. In these trials, subjects were eligible for retreatment no earlier than 12 months after study entry. Over the three year follow up period, approximately 75% of the ILUVIEN treated subjects received only one ILUVIEN implant.
The most common ocular (study eye) and non-ocular adverse reactions are shown in Tables 1 and 2 :
| 1 Includes cataract, cataract nuclear, cataract subcapsular, cataract cortical and cataract diabetic in patients who were phakic at baseline. Among these patients, 80% of ILUVIEN subjects vs. 27% of sham-controlled subjects underwent cataract surgery. | ||||
| 2 235 of the 375 ILUVIEN subjects were phakic at baseline; 121 of 185 sham-controlled subjects were phakic at baseline. | ||||
| Adverse Reactions | ILUVIEN (N=375) n (%) | Sham (N=185) n (%) | ||
| Cataract 1 | 192/235 2 (82%) | 61/121 2 (50%) | ||
| Myodesopsia | 80 (21%) | 17 (9%) | ||
| Eye pain | 57 (15%) | 25 (14%) | ||
| Conjunctival haemorrhage | 50 (13%) | 21 (11%) | ||
| Posterior capsule opacification | 35 (9%) | 6 (3%) | ||
| Eye irritation | 30 (8%) | 11 (6%) | ||
| Vitreous detachment | 26 (7%) | 12 (7%) | ||
| Conjunctivitis | 14 (4%) | 5 (3%) | ||
| Corneal oedema | 13 (4%) | 3 (2%) | ||
| Foreign body sensation in eyes | 12 (3%) | 4 (2%) | ||
| Eye pruritus | 10 (3%) | 3 (2%) | ||
| Ocular hyperaemia | 10 (3%) | 3 (2%) | ||
| Optic atrophy | 9 (2%) | 2 (1%) | ||
| Ocular discomfort | 8 (2%) | 1 (1%) | ||
| Photophobia | 7 (2%) | 2 (1%) | ||
| Retinal exudates | 7 (2%) | 0 (0%) | ||
| Anterior chamber cell | 6 (2%) | 1 (1%) | ||
| Eye discharge | 6 (2%) | 1 (1%) | ||
| Anemia | 40 (11%) | 10 (5%) | ||
| Headache | 33 (9%) | 11 (6%) | ||
| Renal Failure | 32 (9%) | 10 (5%) | ||
| Pneumonia | 28 (7%) | 8 (4%) | ||
| Event | ILUVIEN (N=375) n (%) | Sham (N=185) n (%) |
| IOP elevation ≥ 10 mmHg from Baseline | 127 (34%) | 18 (10%) |
| IOP elevation ≥ 30 mmHg | 75 (20%) | 8 (4%) |
| Any IOP-lowering medication | 144 (38%) | 26 (14%) |
| Any surgical intervention for elevated intraocular pressure | 18 (5%) | 1 (1%) |
Cataracts and Cataract Surgery in DME Patients In the DME studies at baseline, 235 of the 375 ILUVIEN subjects were phakic; 121 of 185 sham-controlled subjects were phakic. The incidence of cataract development in patients who had a phakic study eye was higher in the ILUVIEN group (82%) compared with Sham (50%). The median time of cataract being reported as an adverse event was approximately 12 months in the ILUVIEN group and 19 months in the Sham group. Among these patients, 80% of ILUVIEN subjects vs. 27% of sham-controlled subjects underwent cataract surgery, generally within the first 18 months (Median Month 15 for both ILUVIEN group and for Sham) of the studies.
Chronic Non-Infectious Uveitis Affecting the Posterior Segment of the Eye
Studies 1 and 2 were multicenter, randomized, sham injection-controlled, double-masked trials in which patients with non-infectious uveitis affecting the posterior segment of the eye were treated once with either fluocinolone acetonide intravitreal implant or sham injection, and then received standard care for the duration of the study. Study 3 was a multicenter, randomized, masked trial in which patients with non-infectious uveitis affecting the posterior segment of the eye were all treated once with fluocinolone acetonide intravitreal implant, administered by one of two different applicators, and then received standard care for the duration of the study.
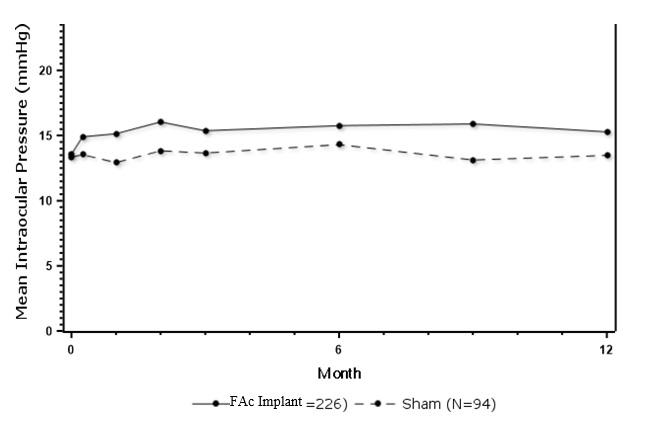
Table 3 summarizes data available from studies 1, 2 and 3 through 12 months for study eyes treated with fluocinolone acetonide intravitreal implant (n=226) or sham injection (n=94). The most common ocular (study eye) and non-ocular adverse reactions in patients with non-infectious uveitis are shown in Table 3 and Table 4 .
| 1 Includes cataract, cataract subcapsular and lenticular opacities in study eyes that were phakic at baseline. 113 of the 226 fluocinolone acetonide study eyes were phakic at baseline; 56 of 94 sham-controlled study eyes were phakic at baseline. | ||
| ADVERSE REACTIONS | Fluocinolone acetonide intravitreal implant (N=226 Eyes) n (%) | Sham Injection (N=94 Eyes) n (%) |
| Cataract 1 | 63/113 (56%) | 13/56 (23%) |
| Visual Acuity Reduced | 33 ( 15%) | 11 (12%) |
| Macular Edema | 25 ( 11%) | 33 (35%) |
| Uveitis | 22 ( 10%) | 33 ( 35%) |
| Conjunctival Hemorrhage | 17 ( 8%) | 5 ( 5%) |
| Eye Pain | 17 ( 8%) | 12 (13%) |
| Hypotony Of Eye | 16 ( 7%) | 1 ( 1%) |
| Anterior Chamber Inflammation | 12 ( 5%) | 6 ( 6%) |
| Dry Eye | 10 ( 4%) | 3 ( 3%) |
| Vitreous Opacities | 9 ( 4%) | 8 ( 9%) |
| Conjunctivitis | 9 ( 4%) | 5 ( 5%) |
| Posterior Capsule Opacification | 8 ( 4%) | 3 ( 3%) |
| Ocular Hyperemia | 8 ( 4%) | 7 ( 7%) |
| Vitreous Haze | 7 ( 3%) | 4 ( 4%) |
| Foreign Body Sensation In Eyes | 7 ( 3%) | 2 ( 2%) |
| Vitritis | 6 ( 3%) | 8 ( 9%) |
| Vitreous Floaters | 6 ( 3%) | 5 ( 5%) |
| Eye Pruritus | 6 ( 3%) | 5 ( 5%) |
| Conjunctival Hyperemia | 5 ( 2%) | 2 ( 2%) |
| Ocular Discomfort | 5 ( 2%) | 1 ( 1%) |
| Macular Fibrosis | 5 ( 2%) | 2 ( 2%) |
| Glaucoma | 4 ( 2%) | 1 ( 1%) |
| Photopsia | 4 ( 2%) | 2 ( 2%) |
| Vitreous Hemorrhage | 4 ( 2%) | 0 |
| Iridocyclitis | 3 ( 1%) | 7 ( 7%) |
| Eye Inflammation | 3 ( 1%) | 2 ( 2%) |
| Choroiditis | 3 ( 1%) | 1 ( 1%) |
| Eye Irritation | 3 ( 1%) | 1 ( 1%) |
| Visual Field Defect | 3 ( 1%) | 0 |
| Lacrimation Increased | 3 ( 1%) | 0 |
| ADVERSE REACTIONS | Fluocinolone acetonide intravitreal implant (N=214 Patients) n (%) | Sham Injection (N=94 Patients) n (%) |
| Nasopharyngitis | 10 ( 5%) | 5 ( 5%) |
| Hypertension | 6 ( 3%) | 1 ( 1%) |
| Arthralgia | 5 ( 2%) | 1 ( 1%) |
| ADVERSE REACTIONS | Fluocinolone acetonide intravitreal implant (N=226 Eyes) n (%) | Sham (N=94 Eyes) n (%) |
| IOP elevation ≥ 10 mmHg from Baseline | 50 (22%) | 11 (12%) |
| IOP elevation > 30 mmHg | 28 (12%) | 3 (3%) |
| Any IOP-lowering medication | 98 (43%) | 39 (41%) |
| Any surgical intervention for elevated IOP | 5 (2%) | 2 (2%) |
Postmarketing Experience
The following adverse reactions have been identified during postapproval use of ILUVIEN. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency or establish a causal relationship to drug exposure. These reactions include reports of drug administration error and reports of the drug being ineffective.
DESCRIPTION
ILUVIEN is a sterile non-bioerodable intravitreal implant containing 0.19 mg (190 mcg) fluocinolone acetonide in a 36-month sustained-release drug delivery system. ILUVIEN is designed to release fluocinolone acetonide at an initial rate of 0.25 mcg/day. ILUVIEN is preloaded into a single-use applicator to facilitate injection of the implant directly into the vitreous. The drug substance is a synthetic corticosteroid, fluocinolone acetonide.
The chemical name for fluocinolone acetonide is (6α,11β, 16α)-6,9-difluoro-11,21-dihydroxy-16,17-[(1-methylethylidene)bis-(oxy)]-pregna-1,4-diene-3,20-dione. Its chemical structure is:
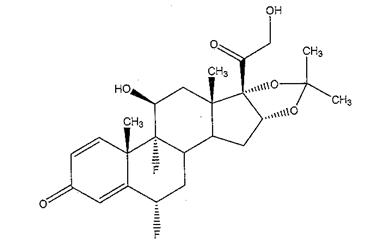
MW 452.50; molecular formula C 24 H 30 F 2 0 6
Fluocinolone acetonide is a white or almost white, microcrystalline powder, practically insoluble in water, soluble in methanol, ethanol, chloroform and acetone, and sparingly soluble in ether.
Each ILUVIEN consists of a light brown 3.5mm x 0.37mm implant containing 0.19 mg of the active ingredient fluocinolone acetonide and the following inactive ingredients: polyimide tube, polyvinyl alcohol, silicone adhesive and water for injection.
CLINICAL PHARMACOLOGY
Mechanism of Action
Corticosteroids inhibit inflammatory responses to a variety of inciting agents including multiple inflammatory cytokines. They inhibit edema, fibrin deposition, capillary dilation, leukocyte migration, capillary proliferation, fibroblast proliferation, deposition of collagen, and scar formation associated with inflammation.
Corticosteroids are thought to act by inhibition of phospholipase A 2 via induction of inhibitory proteins collectively called lipocortins. It is postulated that these proteins control biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting release of the common precursor, arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A 2 .
Pharmacokinetics
In a human pharmacokinetic study of ILUVIEN, fluocinolone acetonide concentrations in plasma were below the lower limit of quantitation of the assay (100 pg/mL) at all post-administration time points from Day 7 through Month 36 following intravitreal administration of a 0.2 mcg/day or 0.5 mcg/day fluocinolone acetonide insert.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been conducted to determine the carcinogenic potential or the effect on fertility of ILUVIEN.
Fluocinolone acetonide was not genotoxic in vitro in the Ames test (S. typhimurium and E. coli) and the mouse lymphoma TK assay, or in vivo in the mouse bone marrow micronucleus assay.
CLINICAL STUDIES
Diabetic Macular Edema
The efficacy of ILUVIEN was assessed in two three year, randomized (2:1, active: sham), multicenter, double-masked, parallel-groups studies that enrolled patients with diabetic macular edema (DME) that had previously been treated with laser photocoagulation.
The primary efficacy endpoint in both trials was the proportion of subjects in whom vision had improved by 15 letters or more from baseline after 24 months of follow-up.
| Study 1 | Study 2 | |||
| ILUVIEN (N=190) | Sham (N=95) | ILUVIEN (N=186) | Sham (N=90) | |
| Mean (SD) Median (Range) | 53 (13) 57 (19-75) | 55 (11) 58 (25-69) | 53 (12) 56 (20-70) | 55 (11) 58 (21-68) |
| a Study 1: ILUVIEN , N=190; Sham, N=95 b Study 2: ILUVIEN , N=186; Sham, N=90 | ||||
| Study | Outcomes | ILUVIEN | Sham | Estimated Difference (95% CI) |
| 1 a | Gain of ≥15 letters in BCVA (n (%)) | 51 (27%) | 14 (15%) | 12.1% (2.6%, 21.6%) |
| Loss of ≥15 letters in BCVA (n (%)) | 26 (14%) | 5 (5%) | 8.4% (1.8%, 15.1%) | |
| Mean change from baseline in BCVA (SD) | 3.7 (18.7) | 3.2 (13.1) | 1.8 (-2.8, 6.3) | |
| 2 b | Gain of ≥15 letters in BCVA (n (%)) | 57 (31%) | 16 (18%) | 13.0% (2.7%, 23.4%) |
| Loss of ≥15 letters in BCVA (n (%)) | 22 (12%) | 9 (10%) | 1.8% (-5.9%, 9.6%) | |
| Mean change from baseline in BCVA (SD) | 5.2 (18.0) | 0.0 (15.6) | 6.1 (1.4, 10.8) | |
Visual acuity outcomes by lens status (Phakic or Pseudophakic) at different visits are presented in Figure 3 and Figure 4 . The occurrence of cataracts impacted visual acuity during the study. Patients who were pseudophakic at baseline achieved greater mean BCVA change from baseline at the Month 24 study visit.
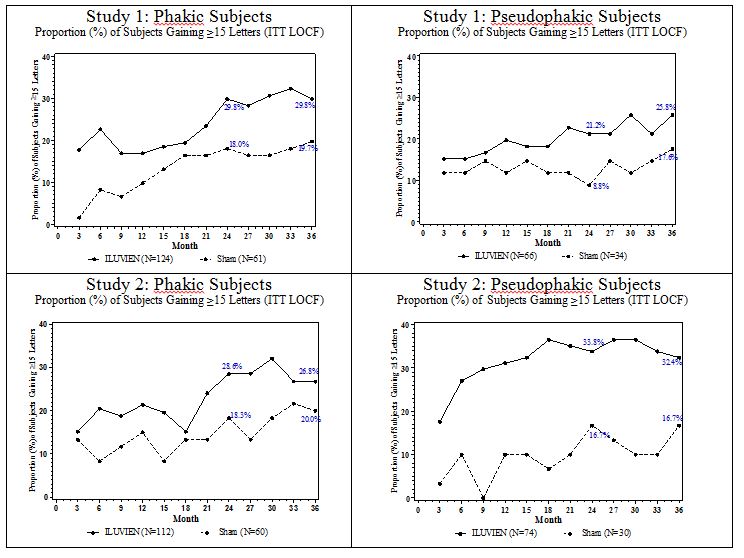
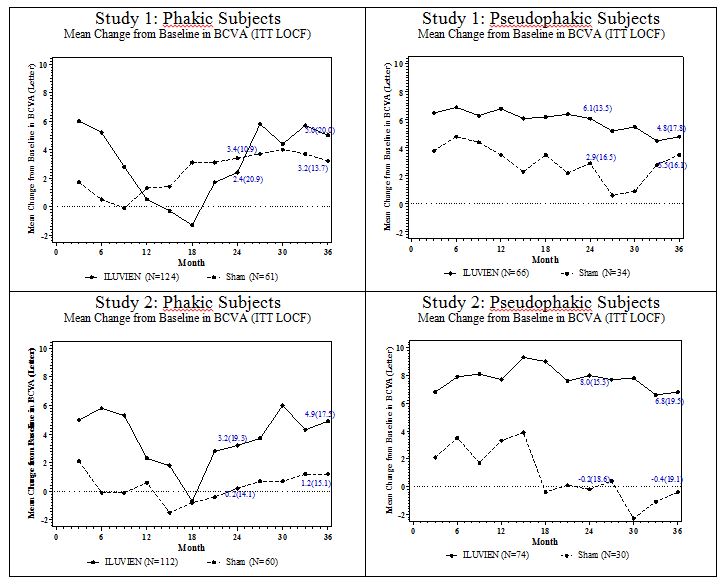
The BCVA outcomes for the Pseudophakic and Phakic subgroups from Studies 1 and 2 at Month 24 are presented in Table 7 .
| a Pseudophakic : ILUVIEN, N=140; Sham, N=64 b Phakic: ILUVIEN, N=236; Sham, N=121 | ||||
| Lens Status | Outcomes | ILUVIEN | Sham | Estimated Difference (95% CI) |
| a Pseudophakic | Gain of ≥15 letters in BCVA (n (%)) | 39 (28%) | 8 (13%) | 15.4% (4.4%, 26.3%) |
| Loss of ≥15 letters in BCVA (n (%)) | 7 (5%) | 7 (11%) | -5.9% (-14.4%, 2.5%) | |
| Mean change from baseline in BCVA (SD) | 7.1 (14.5) | 1.5 (17.4) | 5.6 (0.7, 10.6) | |
| b Phakic | Gain of ≥15 letters in BCVA (n (%)) | 69 (29%) | 22 (18%) | 11.1% (2.1%, 20.1%) |
| Loss of ≥15 letters in BCVA (n (%)) | 41 (17%) | 7 (6%) | 11.6% (5.2%, 18%) | |
| Mean change from baseline in BCVA (SD) | 2.8 (20.1) | 1.8 (12.6) | 1 (-2.5 ,4.4) | |
Chronic Non-Infectious Uveitis Affecting the Posterior Segment
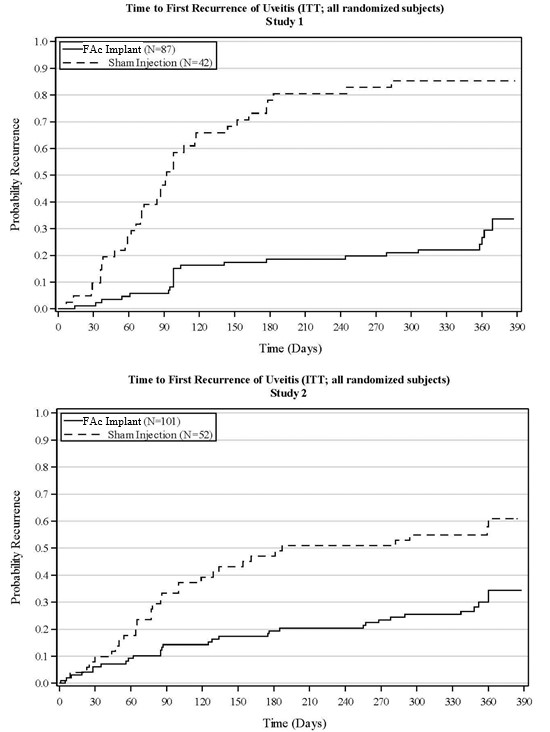
The efficacy of fluocinolone acetonide intravitreal implant was assessed in two randomized (2:1, fluocinolone acetonide intravitreal implant: sham-injection), multi-center, double-masked, parallel-groups studies (NCT #01694186 and #02746991) that enrolled patients with non-infectious uveitis affecting the posterior segment of the eye. The primary efficacy endpoint in both trials was the proportion of patients who experienced a recurrence of uveitis in the study eye within 6 months of follow-up; recurrence was also assessed at 12 months. Recurrence of uveitis was defined as either deterioration in visual acuity, vitreous haze attributable to non-infectious uveitis or the need for rescue medications.
| P-value | < 0.01 | < 0.01 | ||
HOW SUPPLIED/STORAGE AND HANDLING
ILUVIEN® (fluocinolone acetonide intravitreal implant) 0.19 mg is supplied in a sterile, single-use preloaded applicator with a 25-gauge needle, packaged in a tray sealed with a lid inside a carton.
NDC 68611-190-02
Storage: Store at 15°C to 30°C (59°F to 86° F).
Mechanism of Action
Corticosteroids inhibit inflammatory responses to a variety of inciting agents including multiple inflammatory cytokines. They inhibit edema, fibrin deposition, capillary dilation, leukocyte migration, capillary proliferation, fibroblast proliferation, deposition of collagen, and scar formation associated with inflammation.
Corticosteroids are thought to act by inhibition of phospholipase A 2 via induction of inhibitory proteins collectively called lipocortins. It is postulated that these proteins control biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting release of the common precursor, arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A 2 .