Get your patient on Imfinzi (Durvalumab)
Imfinzi prior authorization resources
Most recent state uniform prior authorization forms
Brand Resources
Imfinzi patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
- Administer IMFINZI as an intravenous infusion over 60 minutes after dilution. (2.4 )
- Neoadjuvant and Adjuvant Treatment of Resectable NSCLC:
- Weight ≥ 30 kg:
Neoadjuvant : IMFINZI 1,500 mg in combination with chemotherapy every 3 weeks for up to 4 cycles prior to surgery.
Adjuvant : IMFINZI 1,500 mg as a single agent every 4 weeks for up to 12 cycles after surgery. (2.2 )
- Weight < 30 kg
Neoadjuvant : IMFINZI 20 mg/kg every 3 weeks in combination with chemotherapy for up to 4 cycles prior to surgery.
Adjuvant : 20 mg/kg every 4 weeks as a single agent for up to 12 cycles after surgery. (2.2 )
- Unresectable Stage III NSCLC, following concurrent platinum-based chemotherapy and radiation therapy:
- Metastatic NSCLC:
- Weight ≥ 30 kg: IMFINZI 1,500 mg every 3 weeks in combination with tremelimumab-actl 75 mg and platinum-based chemotherapy for 4 cycles, and then administer IMFINZI 1,500 mg every 4 weeks as a single agent with histology-based pemetrexed maintenance therapy every 4 weeks, and a fifth dose of tremelimumab-actl 75 mg in combination with IMFINZI dose 6 at week 16. (2.2 )
- Weight < 30 kg: IMFINZI 20 mg/kg every 3 weeks in combination with tremelimumab-actl 1 mg/kg and platinum-based chemotherapy, and then administer IMFINZI 20 mg/kg every 4 weeks as a single agent with histology-based pemetrexed therapy every 4 weeks, and a fifth dose of tremelimumab-actl 1 mg/kg in combination with IMFINZI dose 6 at week 16. (2.2 )
- LS-SCLC, following concurrent platinum-based chemotherapy and radiation therapy:
- ES-SCLC:
- Weight ≥ 30 kg: With etoposide and either carboplatin or cisplatin, administer IMFINZI 1,500 mg every 3 weeks in combination with chemotherapy, and then 1,500 mg every 4 weeks as a single agent. (2.2 )
- Weight < 30 kg: With etoposide and either carboplatin or cisplatin, administer IMFINZI 20 mg/kg every 3 weeks in combination with chemotherapy, and then 10 mg/kg every 2 weeks as a single agent. (2.2 )
- BTC:
- uHCC:
- Weight ≥ 30 kg: IMFINZI 1,500 mg in combination with tremelimumab-actl 300 mg as a single dose at Cycle 1/Day 1, followed by IMFINZI as a single agent every 4 weeks. (2.2 )
- Weight < 30 kg: IMFINZI 20 mg/kg in combination with tremelimumab-actl 4 mg/kg as a single dose at Cycle 1/Day 1, followed by IMFINZI as a single agent every 4 weeks. (2.2 )
- dMMR endometrial cancer:
- Weight ≥ 30 kg: IMFINZI 1,120 mg in combination with carboplatin and paclitaxel every 3 weeks for 6 cycles, followed by IMFINZI 1,500 mg every 4 weeks as a single agent. (2.1 , 2.2 )
- Weight < 30 kg: IMFINZI 15 mg/kg in combination with carboplatin and paclitaxel every 3 weeks for 6 cycles, followed by IMFINZI 20 mg/kg every 4 weeks as a single agent. (2.1 , 2.2 )
- MIBC:
- Weight ≥ 30 kg:
Neoadjuvant: IMFINZI 1,500 mg in combination with gemcitabine and cisplatin every 3 weeks for 4 cycles prior to surgery.
Adjuvant: IMFINZI 1,500 mg every 4 weeks as a single agent for up to 8 cycles after surgery. (2.2 )
- Weight < 30 kg:
Neoadjuvant: IMFINZI 20 mg/kg in combination with gemcitabine and cisplatin every 3 weeks for 4 cycles prior to surgery.
Adjuvant: IMFINZI 20 mg/kg every 4 weeks as a single agent for up to 8 cycles after surgery. (2.2 )
- See full Prescribing Information for preparation and administration instructions and dosage modifications for adverse reactions.
Patient Selection
Advanced or Recurrent dMMR Endometrial Cancer
Select patients for treatment based on the presence of dMMR in tumor specimens [see Clinical Studies (14.5) ].
Information on FDA-approved tests for the detection of dMMR status in endometrial cancer is available at https://www.fda.gov/companiondiagnostics .
Recommended Dosage
The recommended dosages for IMFINZI as a single agent and IMFINZI in combination with other therapeutic agents are presented in Table 1. The recommended dosage schedule and regimens for IMFINZI for the treatment of metastatic NSCLC are provided in Tables 2 and 3 [see Indications and Usage (1.1) ].
Administer IMFINZI as a 60 minute intravenous infusion after dilution [see Dosage and Administration (2.3) ].
| Indication | Recommended IMFINZI Dosage | Duration of Therapy |
|---|---|---|
| Neoadjuvant and Adjuvant Treatment of Resectable NSCLC | Patients with a body weight of ≥ 30 kg: Neoadjuvant: IMFINZI 1,500 mg in combination with chemotherapy Administer IMFINZI prior to chemotherapy on the same day. Refer to the Prescribing Information for the agent administered in combination with IMFINZI for recommended dosage information, as appropriate. every 3 weeks for up to 4 cycles prior to surgery Adjuvant: IMFINZI 1,500 mg as a single agent every 4 weeks for up to 12 cycles after surgery. Patients with a body weight of < 30 kg: Neoadjuvant: IMFINZI 20 mg/kg every 3 weeks in combination with chemotherapyfor up to 4 cycles prior to surgery. Adjuvant: IMFINZI 20 mg/kg every 4 weeks for up to 12 cycles as a single agent after surgery. | Until disease progression that precludes definitive surgery, recurrence, unacceptable toxicity, or a maximum of 12 cycles after surgery |
| Unresectable Stage III NSCLC | Following concurrent platinum-based chemotherapy and radiation therapy: Patients with a body weight of ≥ 30 kg: 10 mg/kg every 2 weeks or 1,500 mg every 4 weeks Patients with a body weight of < 30 kg: 10 mg/kg every 2 weeks | Until disease progression, unacceptable toxicity, or a maximum of 12 months |
| Limited Stage SCLC | Following concurrent platinum-based chemotherapy and radiation therapy: Patients with a body weight of ≥ 30 kg: 1,500 mg every 4 weeks Patients with a body weight of < 30 kg: 20 mg/kg every 4 weeks | Until disease progression, unacceptable toxicity, or a maximum of 24 months |
| Extensive Stage SCLC | Patients with a body weight of ≥ 30 kg: 1,500 mg in combination with chemotherapyevery 3 weeks (21 days) for 4 cycles, followed by 1,500 mg every 4 weeks as a single agent Patients with a body weight of < 30 kg: 20 mg/kg in combination with chemotherapyevery 3 weeks (21 days) for 4 cycles, followed by 10 mg/kg every 2 weeks as a single agent | Until disease progression or until unacceptable toxicity |
| BTC | Patients with a body weight of ≥ 30 kg: 1,500 mg in combination with chemotherapyevery 3 weeks (21 days) up to 8 cycles followed by 1,500 mg every 4 weeks as a single agent Patients with a body weight of < 30 kg: 20 mg/kg in combination with chemotherapyevery 3 weeks (21 days) up to 8 cycles, followed by 20 mg/kg every 4 weeks as a single agent | Until disease progression or until unacceptable toxicity |
| uHCC | Patients with a body weight of ≥ 30 kg: IMFINZI 1,500 mg following a single dose of tremelimumab-actl Administer tremelimumab-actl prior to IMFINZI on the same day. When tremelimumab-actl is administered in combination with IMFINZI, refer to the Prescribing Information for tremelimumab-actl dosing information. 300 mg at Day 1 of Cycle 1; Continue IMFINZI 1,500 mg as a single agent every 4 weeks Patients with a body weight of < 30 kg: IMFINZI 20 mg/kg following a single dose of tremelimumab-actl4 mg/kg at Day 1 of Cycle 1; Continue IMFINZI 20 mg/kg as a single agent every 4 weeks | After Cycle 1 of combination therapy, administer IMFINZI as a single agent every 4 weeks until disease progression or unacceptable toxicity |
| dMMR endometrial cancer | Patients with a body weight of ≥ 30 kg: IMFINZI 1,120 mg in combination with carboplatin and paclitaxel every 3 weeks (21 days) for 6 cycles, followed by IMFINZI 1,500 mg every 4 weeks as a single agent Patients with a body weight of < 30 kg: IMFINZI 15 mg/kg in combination with carboplatin and paclitaxel every 3 weeks (21 days) for 6 cycles, followed by IMFINZI 20 mg/kg every 4 weeks as a single agent | Until disease progression or unacceptable toxicity |
| Neoadjuvant and Adjuvant Treatment of MIBC | Patients with a body weight of ≥30 kg: Neoadjuvant: 1,500 mg in combination with gemcitabine and cisplatin every 3 weeks for 4 cycles prior to surgery Adjuvant: IMFINZI 1,500 mg as a single agent every 4 weeks for up to 8 cycles after surgery Patients with a body weight of < 30 kg: Neoadjuvant: 20 mg/kg in combination with gemcitabine and cisplatin every 3 weeks for 4 cycles prior to surgery Adjuvant: IMFINZI 20 mg/kg as a single agent every 4 weeks for up to 8 cycles after surgery | Until disease progression that precludes definitive surgery, recurrence, or unacceptable toxicity or a maximum of 8 cycles after surgery |
| Neoadjuvant and Adjuvant Treatment of Resectable GC/GEJC | Patients with a body weight of ≥ 30 kg: Neoadjuvant: IMFINZI 1,500 mg every 4 weeks with FLOT FLOT chemotherapy is administered on days 1 and 15 of each 4 week cycle [See Clinical Studies (14.7 )] . for up to 2 cycles prior to surgery Adjuvant: IMFINZI 1,500 mg every 4 weeks with FLOT for up to 2 cycles, followed by IMFINZI 1,500 mg as a single agent every 4 weeks for up to 10 cycles Patients with a body weight of < 30 kg: Neoadjuvant: IMFINZI 20 mg/kg every 4 weeks with FLOT•± for up to 2 cycles prior to surgery Adjuvant: IMFINZI 20 mg/kg every 4 weeks with FLOT for up to 2 cycles, followed by IMFINZI 20 mg/kg as a single agent every 4 weeks for up to 10 cycles | Neoadjuvant: Until disease progression that precludes definitive surgery or unacceptable toxicity |
IMFINZI in Combination with Tremelimumab-actl and Platinum-Based Chemotherapy
The recommended dosage schedule and regimens for IMFINZI for the treatment of metastatic NSCLC are provided in Tables 2 and 3.
Weigh patients prior to each infusion.
Calculate the appropriate dose using Table 3 below based on the patient’s weight and tumor histology.
| Week continue IMFINZI until disease progression or intolerable toxicity. note the dosing interval change from every 3 weeks to every 4 weeks starting at cycle 5. | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 |
| Cycle: | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||||||||||||||||
IMFINZI intravenous infusion over 60 minutes [see Dosage and Administration (2.4) ] . | X | X | X | X | X | X | X | X | ||||||||||||||||
Tremelimumab-actl if patients receive fewer than 4 cycles of platinum-based chemotherapy, the remaining cycles of tremelimumab-actl (up to a total of 5) should be given after the platinum-based chemotherapy phase, in combination with IMFINZI, every 4 weeks. | X | X | X | X | X | |||||||||||||||||||
Chemotherapy | X | X | X | X | X optional pemetrexed therapy from week 12 until disease progression or intolerable toxicity for patients with non-squamous disease who received treatment with pemetrexed and carboplatin/cisplatin. | X | X | X | ||||||||||||||||
| Tumor Histology | Patient Weight | IMFINZI Dosage | Tremelimumab-actl Dosage Refer to the Prescribing Information for dosing information. | Platinum-based Chemotherapy Regimen |
|---|---|---|---|---|
Non-Squamous | ≥ 30 kg | 1,500 mg | 75 mg |
OR
|
< 30 kg | 20 mg/kg | 1 mg/kg | ||
Squamous | ≥ 30 kg | 1,500 mg | 75 mg |
OR
|
< 30 kg | 20 mg/kg | 1 mg/kg |
Dosage Modifications for Adverse Reactions
No dose reduction for IMFINZI is recommended. In general, withhold IMFINZI for severe (Grade 3) immune-mediated adverse reactions. Permanently discontinue IMFINZI for life-threatening (Grade 4) immune-mediated adverse reactions, recurrent severe (Grade 3) immune-mediated reactions that require systemic immunosuppressive treatment, or an inability to reduce corticosteroid dose to 10 mg or less of prednisone or equivalent per day within 12 weeks of initiating corticosteroids.
Dosage modifications for IMFINZI or IMFINZI in combination with tremelimumab-actl or chemotherapy for adverse reactions that require management different from these general guidelines are summarized in Table 4.
| Adverse Reaction | Severity Based on National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. | Dosage Modification |
|---|---|---|
Immune-Mediated Adverse Reactions [see Warnings and Precautions (5.1) ] | ||
| Grade 2 | Withhold Resume in patients with complete or partial resolution (Grade 0 to 1) after corticosteroid taper. Permanently discontinue if no complete or partial resolution within 12 weeks of initiating corticosteroids or an inability to reduce corticosteroid dose to 10 mg of prednisone or less per day (or equivalent) within 12 weeks of initiating corticosteroids. |
Grade 3 or 4 | Permanently discontinue | |
| Grade 2 | Withhold |
Grade 3 | Withholdor permanently discontinue Permanently discontinue IMFINZI for Grade 3 colitis when administered as part of a tremelimumab-actl containing regimen. | |
Grade 4 | Permanently discontinue | |
| Any grade | Permanently discontinue |
| ALT or AST increases to more than 3 and up to 8 times the ULN or total bilirubin increases to more than 1.5 and up to 3 times ULN | Withhold |
ALT or AST increases to more than 8 times ULN or total bilirubin increases to more than 3 times the ULN | Permanently discontinue | |
| AST or ALT is more than 1 and up to 3 times ULN at baseline and increases to more than 5 and up to 10 times ULN or AST or ALT is more than 3 and up to 5 times ULN at baseline and increases to more than 8 and up to 10 times ULN | Withhold |
AST or ALT increases to more than 10 times ULN or total bilirubin increases to more than 3 times ULN | Permanently discontinue | |
| Grade 3 or 4 | Withhold until clinically stable or permanently discontinue depending on severity |
| Grade 2 or 3 increased blood creatinine | Withhold |
Grade 4 increased blood creatinine | Permanently discontinue | |
| Suspected SJS, TEN, or DRESS | Withhold |
Confirmed SJS, TEN, or DRESS | Permanently discontinue | |
| Grade 2, 3, or 4 | Permanently discontinue |
| Grade 2 | Withhold |
Grade 3 or 4 | Permanently discontinue | |
Other Adverse Reactions | ||
| Grade 1 or 2 | Interrupt or slow the rate of infusion |
Grade 3 or 4 | Permanently discontinue | |
ALT = alanine aminotransferase, AST = aspartate aminotransferase, DRESS = Drug Rash with Eosinophilia and Systemic Symptoms, SJS = Stevens Johnson Syndrome, TEN = toxic epidermal necrolysis, ULN = upper limit normal. | ||
Preparation and Administration
Preparation
- Visually inspect drug product for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard the vial if the solution is cloudy, discolored, or visible particles are observed.
- Do not shake the vial.
- Withdraw the required volume from the vial(s) of IMFINZI and transfer into an intravenous bag containing 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP. Mix diluted solution by gentle inversion. Do not shake the solution. The final concentration of the diluted solution should be between 1 mg/mL and 15 mg/mL.
- Discard partially used or empty vials of IMFINZI.
Storage of Infusion Solution
- IMFINZI does not contain a preservative.
- Administer infusion solution immediately once prepared. If the infusion solution is not administered immediately and needs to be stored, the time from preparation until the completion of the infusion should not exceed:
- 28 days in a refrigerator at 2°C to 8°C (36°F to 46°F)
- 8 hours at room temperature up to 25°C (77°F)
- Do not freeze.
- Do not shake.
Administration
- Administer infusion solution intravenously over 60 minutes through an intravenous line containing a sterile, low-protein binding 0.2 or 0.22 micron in-line filter.
- Use separate infusion bags and filters for each drug product.
IMFINZI in Combination with Other Products
- Administer all intravenous drug products as separate infusions.
- Do not co-administer other intravenous drugs through the same infusion line.
- For platinum-based chemotherapy, refer to Prescribing Information for administration information.
- For pemetrexed therapy, refer to Prescribing Information for administration information.
Combination Regimens: Order of Infusions
IMFINZI in Combination with Tremelimumab-actl
- Infuse tremelimumab-actl first, followed by IMFINZI on the same day of dosing.
IMFINZI in Combination with Tremelimumab-actl and Platinum-Based Chemotherapy
- Infuse tremelimumab-actl first, followed by IMFINZI and then platinum-based chemotherapy on the day of dosing.
IMFINZI in Combination with Tremelimumab-actl and Pemetrexed Therapy
- Infuse tremelimumab-actl first, followed by IMFINZI and then pemetrexed therapy on the day of dosing.
IMFINZI in Combination with Chemotherapy
- Infuse IMFINZI first and then chemotherapy on the same day of dosing.
Combination Regimens: Infusion Instructions
IMFINZI in Combination with Tremelimumab-actl
- Administer tremelimumab-actl over 60 minutes followed by a 60 minute observation period. Then administer IMFINZI as a separate intravenous infusion over 60 minutes.
IMFINZI in Combination with Tremelimumab-actl and Platinum-Based Chemotherapy/ Pemetrexed Therapy
Cycle 1
- Infuse tremelimumab-actl over 60 minutes. One to two hours after completion of tremelimumab-actl infusion, infuse IMFINZI over 60 minutes. One to two hours after completion of IMFINZI infusion, administer platinum-based chemotherapy.
Subsequent Cycles
- If there are no infusion reactions during cycle 1, subsequent cycles of IMFINZI can be given immediately after tremelimumab-actl. The time between the end of the IMFINZI infusion and the start of chemotherapy can be reduced to 30 minutes.

By using PrescriberAI, you agree to the AI Terms of Use.
Imfinzi prescribing information
Indications and Usage (1.1 , 1.2 ) 12/2024
Indications and Usage (1.5 ) 02/2025
Indications and Usage (1.6 ) 03/2025
Indications and Usage (1.7 ) 11/2025
Dosage and Administration (2.1 ) 02/2025
Dosage and Administration (2.1 , 2.2 , 2.3 , 2.4 ) 12/2024
Dosage and Administration (2.2 , 2.4 ) 03/2025
Dosage and Administration (2.2 ) 11/2025
Warnings and Precautions (5.1 ) 12/2024
INDICATIONS AND USAGE
- IMFINZI is a programmed death-ligand 1 (PD-L1) blocking antibody indicated:
- in combination with platinum-containing chemotherapy as neoadjuvant treatment, followed by IMFINZI continued as a single agent as adjuvant treatment after surgery, for the treatment of adult patients with resectable (tumors ≥ 4 cm and/or node positive) non-small cell lung cancer (NSCLC) and no known epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) rearrangements. (1.1 )
- as a single agent, for the treatment of adult patients with unresectable, Stage III NSCLC whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy. (1.1 )
- in combination with tremelimumab-actl and platinum-based chemotherapy, for the treatment of adult patients with metastatic NSCLC with no sensitizing EGFR mutations or ALK genomic tumor aberrations. (1.1 )
- as a single agent, for the treatment of adult patients with limited-stage small cell lung cancer (LS-SCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy. (1.2 )
- in combination with etoposide and either carboplatin or cisplatin, as first-line treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC). (1.2 )
- in combination with gemcitabine and cisplatin, as treatment of adult patients with locally advanced or metastatic biliary tract cancer (BTC). (1.3 )
- in combination with tremelimumab-actl, for the treatment of adult patients with unresectable hepatocellular carcinoma (uHCC). (1.4 )
- in combination with carboplatin and paclitaxel followed by IMFINZI as a single agent, for the treatment of adult patients with primary advanced or recurrent endometrial cancer that is mismatch repair deficient (dMMR) as determined by an FDA-approved test. (1.5 , 2.1 )
- in combination with gemcitabine and cisplatin as neoadjuvant treatment, followed by single agent IMFINZI as adjuvant treatment following radical cystectomy, for the treatment of adult patients with muscle invasive bladder cancer (MIBC). (1.6 )
- in combination with fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) chemotherapy as neoadjuvant and adjuvant treatment, followed by single agent IMFINZI, for the treatment of adult patients with resectable gastric or gastroesophageal junction adenocarcinoma (GC/GEJC). (1.7 )
Non-Small Cell Lung Cancer
- IMFINZI in combination with platinum-containing chemotherapy as neoadjuvant treatment, followed by IMFINZI continued as a single agent as adjuvant treatment after surgery, is indicated for the treatment of adult patients with resectable (tumors ≥ 4 cm and/or node positive) non-small cell lung cancer (NSCLC) and no known epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) rearrangements.
- IMFINZI, as a single agent, is indicated for the treatment of adult patients with unresectable Stage III NSCLC whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy (cCRT).
- IMFINZI, in combination with tremelimumab-actl and platinum-based chemotherapy, is indicated for the treatment of adult patients with metastatic NSCLC with no sensitizing EGFR mutations or ALK genomic tumor aberrations.
Small Cell Lung Cancer
- IMFINZI, as a single agent, is indicated for the treatment of adult patients with limited-stage small cell lung cancer (LS-SCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy (cCRT).
- IMFINZI, in combination with etoposide and either carboplatin or cisplatin, is indicated for the first-line treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC).
Biliary Tract Cancers
IMFINZI, in combination with gemcitabine and cisplatin, is indicated for the treatment of adult patients with locally advanced or metastatic biliary tract cancer (BTC).
Hepatocellular Carcinoma
IMFINZI, in combination with tremelimumab-actl, is indicated for the treatment of adult patients with unresectable hepatocellular carcinoma (uHCC).
Endometrial Cancer
IMFINZI, in combination with carboplatin and paclitaxel followed by IMFINZI as a single agent, is indicated for the treatment of adult patients with primary advanced or recurrent endometrial cancer that is mismatch repair deficient (dMMR) as determined by an FDA-approved test [see Dosage and Administration (2.1)].
Bladder Cancer
IMFINZI in combination with gemcitabine and cisplatin as neoadjuvant treatment, followed by single agent IMFINZI as adjuvant treatment following radical cystectomy, is indicated for the treatment of adult patients with muscle invasive bladder cancer (MIBC).
Gastric or gastroesophageal junction adenocarcinoma
IMFINZI in combination with fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) as neoadjuvant and adjuvant treatment, followed by single-agent IMFINZI, is indicated for the treatment of adult patients with resectable gastric or gastroesophageal junction adenocarcinoma (GC/GEJC).
DOSAGE AND ADMINISTRATION
- Administer IMFINZI as an intravenous infusion over 60 minutes after dilution. (2.4 )
- Neoadjuvant and Adjuvant Treatment of Resectable NSCLC:
- Weight ≥ 30 kg:
Neoadjuvant : IMFINZI 1,500 mg in combination with chemotherapy every 3 weeks for up to 4 cycles prior to surgery.
Adjuvant : IMFINZI 1,500 mg as a single agent every 4 weeks for up to 12 cycles after surgery. (2.2 )
- Weight < 30 kg
Neoadjuvant : IMFINZI 20 mg/kg every 3 weeks in combination with chemotherapy for up to 4 cycles prior to surgery.
Adjuvant : 20 mg/kg every 4 weeks as a single agent for up to 12 cycles after surgery. (2.2 )
- Unresectable Stage III NSCLC, following concurrent platinum-based chemotherapy and radiation therapy:
- Metastatic NSCLC:
- Weight ≥ 30 kg: IMFINZI 1,500 mg every 3 weeks in combination with tremelimumab-actl 75 mg and platinum-based chemotherapy for 4 cycles, and then administer IMFINZI 1,500 mg every 4 weeks as a single agent with histology-based pemetrexed maintenance therapy every 4 weeks, and a fifth dose of tremelimumab-actl 75 mg in combination with IMFINZI dose 6 at week 16. (2.2 )
- Weight < 30 kg: IMFINZI 20 mg/kg every 3 weeks in combination with tremelimumab-actl 1 mg/kg and platinum-based chemotherapy, and then administer IMFINZI 20 mg/kg every 4 weeks as a single agent with histology-based pemetrexed therapy every 4 weeks, and a fifth dose of tremelimumab-actl 1 mg/kg in combination with IMFINZI dose 6 at week 16. (2.2 )
- LS-SCLC, following concurrent platinum-based chemotherapy and radiation therapy:
- ES-SCLC:
- Weight ≥ 30 kg: With etoposide and either carboplatin or cisplatin, administer IMFINZI 1,500 mg every 3 weeks in combination with chemotherapy, and then 1,500 mg every 4 weeks as a single agent. (2.2 )
- Weight < 30 kg: With etoposide and either carboplatin or cisplatin, administer IMFINZI 20 mg/kg every 3 weeks in combination with chemotherapy, and then 10 mg/kg every 2 weeks as a single agent. (2.2 )
- BTC:
- uHCC:
- Weight ≥ 30 kg: IMFINZI 1,500 mg in combination with tremelimumab-actl 300 mg as a single dose at Cycle 1/Day 1, followed by IMFINZI as a single agent every 4 weeks. (2.2 )
- Weight < 30 kg: IMFINZI 20 mg/kg in combination with tremelimumab-actl 4 mg/kg as a single dose at Cycle 1/Day 1, followed by IMFINZI as a single agent every 4 weeks. (2.2 )
- dMMR endometrial cancer:
- Weight ≥ 30 kg: IMFINZI 1,120 mg in combination with carboplatin and paclitaxel every 3 weeks for 6 cycles, followed by IMFINZI 1,500 mg every 4 weeks as a single agent. (2.1 , 2.2 )
- Weight < 30 kg: IMFINZI 15 mg/kg in combination with carboplatin and paclitaxel every 3 weeks for 6 cycles, followed by IMFINZI 20 mg/kg every 4 weeks as a single agent. (2.1 , 2.2 )
- MIBC:
- Weight ≥ 30 kg:
Neoadjuvant: IMFINZI 1,500 mg in combination with gemcitabine and cisplatin every 3 weeks for 4 cycles prior to surgery.
Adjuvant: IMFINZI 1,500 mg every 4 weeks as a single agent for up to 8 cycles after surgery. (2.2 )
- Weight < 30 kg:
Neoadjuvant: IMFINZI 20 mg/kg in combination with gemcitabine and cisplatin every 3 weeks for 4 cycles prior to surgery.
Adjuvant: IMFINZI 20 mg/kg every 4 weeks as a single agent for up to 8 cycles after surgery. (2.2 )
- See full Prescribing Information for preparation and administration instructions and dosage modifications for adverse reactions.
Patient Selection
Advanced or Recurrent dMMR Endometrial Cancer
Select patients for treatment based on the presence of dMMR in tumor specimens [see Clinical Studies (14.5) ].
Information on FDA-approved tests for the detection of dMMR status in endometrial cancer is available at https://www.fda.gov/companiondiagnostics .
Recommended Dosage
The recommended dosages for IMFINZI as a single agent and IMFINZI in combination with other therapeutic agents are presented in Table 1. The recommended dosage schedule and regimens for IMFINZI for the treatment of metastatic NSCLC are provided in Tables 2 and 3 [see Indications and Usage (1.1) ].
Administer IMFINZI as a 60 minute intravenous infusion after dilution [see Dosage and Administration (2.3) ].
| Indication | Recommended IMFINZI Dosage | Duration of Therapy |
|---|---|---|
| Neoadjuvant and Adjuvant Treatment of Resectable NSCLC | Patients with a body weight of ≥ 30 kg: Neoadjuvant: IMFINZI 1,500 mg in combination with chemotherapy Administer IMFINZI prior to chemotherapy on the same day. Refer to the Prescribing Information for the agent administered in combination with IMFINZI for recommended dosage information, as appropriate. every 3 weeks for up to 4 cycles prior to surgery Adjuvant: IMFINZI 1,500 mg as a single agent every 4 weeks for up to 12 cycles after surgery. Patients with a body weight of < 30 kg: Neoadjuvant: IMFINZI 20 mg/kg every 3 weeks in combination with chemotherapyfor up to 4 cycles prior to surgery. Adjuvant: IMFINZI 20 mg/kg every 4 weeks for up to 12 cycles as a single agent after surgery. | Until disease progression that precludes definitive surgery, recurrence, unacceptable toxicity, or a maximum of 12 cycles after surgery |
| Unresectable Stage III NSCLC | Following concurrent platinum-based chemotherapy and radiation therapy: Patients with a body weight of ≥ 30 kg: 10 mg/kg every 2 weeks or 1,500 mg every 4 weeks Patients with a body weight of < 30 kg: 10 mg/kg every 2 weeks | Until disease progression, unacceptable toxicity, or a maximum of 12 months |
| Limited Stage SCLC | Following concurrent platinum-based chemotherapy and radiation therapy: Patients with a body weight of ≥ 30 kg: 1,500 mg every 4 weeks Patients with a body weight of < 30 kg: 20 mg/kg every 4 weeks | Until disease progression, unacceptable toxicity, or a maximum of 24 months |
| Extensive Stage SCLC | Patients with a body weight of ≥ 30 kg: 1,500 mg in combination with chemotherapyevery 3 weeks (21 days) for 4 cycles, followed by 1,500 mg every 4 weeks as a single agent Patients with a body weight of < 30 kg: 20 mg/kg in combination with chemotherapyevery 3 weeks (21 days) for 4 cycles, followed by 10 mg/kg every 2 weeks as a single agent | Until disease progression or until unacceptable toxicity |
| BTC | Patients with a body weight of ≥ 30 kg: 1,500 mg in combination with chemotherapyevery 3 weeks (21 days) up to 8 cycles followed by 1,500 mg every 4 weeks as a single agent Patients with a body weight of < 30 kg: 20 mg/kg in combination with chemotherapyevery 3 weeks (21 days) up to 8 cycles, followed by 20 mg/kg every 4 weeks as a single agent | Until disease progression or until unacceptable toxicity |
| uHCC | Patients with a body weight of ≥ 30 kg: IMFINZI 1,500 mg following a single dose of tremelimumab-actl Administer tremelimumab-actl prior to IMFINZI on the same day. When tremelimumab-actl is administered in combination with IMFINZI, refer to the Prescribing Information for tremelimumab-actl dosing information. 300 mg at Day 1 of Cycle 1; Continue IMFINZI 1,500 mg as a single agent every 4 weeks Patients with a body weight of < 30 kg: IMFINZI 20 mg/kg following a single dose of tremelimumab-actl4 mg/kg at Day 1 of Cycle 1; Continue IMFINZI 20 mg/kg as a single agent every 4 weeks | After Cycle 1 of combination therapy, administer IMFINZI as a single agent every 4 weeks until disease progression or unacceptable toxicity |
| dMMR endometrial cancer | Patients with a body weight of ≥ 30 kg: IMFINZI 1,120 mg in combination with carboplatin and paclitaxel every 3 weeks (21 days) for 6 cycles, followed by IMFINZI 1,500 mg every 4 weeks as a single agent Patients with a body weight of < 30 kg: IMFINZI 15 mg/kg in combination with carboplatin and paclitaxel every 3 weeks (21 days) for 6 cycles, followed by IMFINZI 20 mg/kg every 4 weeks as a single agent | Until disease progression or unacceptable toxicity |
| Neoadjuvant and Adjuvant Treatment of MIBC | Patients with a body weight of ≥30 kg: Neoadjuvant: 1,500 mg in combination with gemcitabine and cisplatin every 3 weeks for 4 cycles prior to surgery Adjuvant: IMFINZI 1,500 mg as a single agent every 4 weeks for up to 8 cycles after surgery Patients with a body weight of < 30 kg: Neoadjuvant: 20 mg/kg in combination with gemcitabine and cisplatin every 3 weeks for 4 cycles prior to surgery Adjuvant: IMFINZI 20 mg/kg as a single agent every 4 weeks for up to 8 cycles after surgery | Until disease progression that precludes definitive surgery, recurrence, or unacceptable toxicity or a maximum of 8 cycles after surgery |
| Neoadjuvant and Adjuvant Treatment of Resectable GC/GEJC | Patients with a body weight of ≥ 30 kg: Neoadjuvant: IMFINZI 1,500 mg every 4 weeks with FLOT FLOT chemotherapy is administered on days 1 and 15 of each 4 week cycle [See Clinical Studies (14.7 )] . for up to 2 cycles prior to surgery Adjuvant: IMFINZI 1,500 mg every 4 weeks with FLOT for up to 2 cycles, followed by IMFINZI 1,500 mg as a single agent every 4 weeks for up to 10 cycles Patients with a body weight of < 30 kg: Neoadjuvant: IMFINZI 20 mg/kg every 4 weeks with FLOT•± for up to 2 cycles prior to surgery Adjuvant: IMFINZI 20 mg/kg every 4 weeks with FLOT for up to 2 cycles, followed by IMFINZI 20 mg/kg as a single agent every 4 weeks for up to 10 cycles | Neoadjuvant: Until disease progression that precludes definitive surgery or unacceptable toxicity |
IMFINZI in Combination with Tremelimumab-actl and Platinum-Based Chemotherapy
The recommended dosage schedule and regimens for IMFINZI for the treatment of metastatic NSCLC are provided in Tables 2 and 3.
Weigh patients prior to each infusion.
Calculate the appropriate dose using Table 3 below based on the patient’s weight and tumor histology.
| Week continue IMFINZI until disease progression or intolerable toxicity. note the dosing interval change from every 3 weeks to every 4 weeks starting at cycle 5. | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 |
| Cycle: | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||||||||||||||||
IMFINZI intravenous infusion over 60 minutes [see Dosage and Administration (2.4) ] . | X | X | X | X | X | X | X | X | ||||||||||||||||
Tremelimumab-actl if patients receive fewer than 4 cycles of platinum-based chemotherapy, the remaining cycles of tremelimumab-actl (up to a total of 5) should be given after the platinum-based chemotherapy phase, in combination with IMFINZI, every 4 weeks. | X | X | X | X | X | |||||||||||||||||||
Chemotherapy | X | X | X | X | X optional pemetrexed therapy from week 12 until disease progression or intolerable toxicity for patients with non-squamous disease who received treatment with pemetrexed and carboplatin/cisplatin. | X | X | X | ||||||||||||||||
| Tumor Histology | Patient Weight | IMFINZI Dosage | Tremelimumab-actl Dosage Refer to the Prescribing Information for dosing information. | Platinum-based Chemotherapy Regimen |
|---|---|---|---|---|
Non-Squamous | ≥ 30 kg | 1,500 mg | 75 mg |
OR
|
< 30 kg | 20 mg/kg | 1 mg/kg | ||
Squamous | ≥ 30 kg | 1,500 mg | 75 mg |
OR
|
< 30 kg | 20 mg/kg | 1 mg/kg |
Dosage Modifications for Adverse Reactions
No dose reduction for IMFINZI is recommended. In general, withhold IMFINZI for severe (Grade 3) immune-mediated adverse reactions. Permanently discontinue IMFINZI for life-threatening (Grade 4) immune-mediated adverse reactions, recurrent severe (Grade 3) immune-mediated reactions that require systemic immunosuppressive treatment, or an inability to reduce corticosteroid dose to 10 mg or less of prednisone or equivalent per day within 12 weeks of initiating corticosteroids.
Dosage modifications for IMFINZI or IMFINZI in combination with tremelimumab-actl or chemotherapy for adverse reactions that require management different from these general guidelines are summarized in Table 4.
| Adverse Reaction | Severity Based on National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. | Dosage Modification |
|---|---|---|
Immune-Mediated Adverse Reactions [see Warnings and Precautions (5.1) ] | ||
| Grade 2 | Withhold Resume in patients with complete or partial resolution (Grade 0 to 1) after corticosteroid taper. Permanently discontinue if no complete or partial resolution within 12 weeks of initiating corticosteroids or an inability to reduce corticosteroid dose to 10 mg of prednisone or less per day (or equivalent) within 12 weeks of initiating corticosteroids. |
Grade 3 or 4 | Permanently discontinue | |
| Grade 2 | Withhold |
Grade 3 | Withholdor permanently discontinue Permanently discontinue IMFINZI for Grade 3 colitis when administered as part of a tremelimumab-actl containing regimen. | |
Grade 4 | Permanently discontinue | |
| Any grade | Permanently discontinue |
| ALT or AST increases to more than 3 and up to 8 times the ULN or total bilirubin increases to more than 1.5 and up to 3 times ULN | Withhold |
ALT or AST increases to more than 8 times ULN or total bilirubin increases to more than 3 times the ULN | Permanently discontinue | |
| AST or ALT is more than 1 and up to 3 times ULN at baseline and increases to more than 5 and up to 10 times ULN or AST or ALT is more than 3 and up to 5 times ULN at baseline and increases to more than 8 and up to 10 times ULN | Withhold |
AST or ALT increases to more than 10 times ULN or total bilirubin increases to more than 3 times ULN | Permanently discontinue | |
| Grade 3 or 4 | Withhold until clinically stable or permanently discontinue depending on severity |
| Grade 2 or 3 increased blood creatinine | Withhold |
Grade 4 increased blood creatinine | Permanently discontinue | |
| Suspected SJS, TEN, or DRESS | Withhold |
Confirmed SJS, TEN, or DRESS | Permanently discontinue | |
| Grade 2, 3, or 4 | Permanently discontinue |
| Grade 2 | Withhold |
Grade 3 or 4 | Permanently discontinue | |
Other Adverse Reactions | ||
| Grade 1 or 2 | Interrupt or slow the rate of infusion |
Grade 3 or 4 | Permanently discontinue | |
ALT = alanine aminotransferase, AST = aspartate aminotransferase, DRESS = Drug Rash with Eosinophilia and Systemic Symptoms, SJS = Stevens Johnson Syndrome, TEN = toxic epidermal necrolysis, ULN = upper limit normal. | ||
Preparation and Administration
Preparation
- Visually inspect drug product for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard the vial if the solution is cloudy, discolored, or visible particles are observed.
- Do not shake the vial.
- Withdraw the required volume from the vial(s) of IMFINZI and transfer into an intravenous bag containing 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP. Mix diluted solution by gentle inversion. Do not shake the solution. The final concentration of the diluted solution should be between 1 mg/mL and 15 mg/mL.
- Discard partially used or empty vials of IMFINZI.
Storage of Infusion Solution
- IMFINZI does not contain a preservative.
- Administer infusion solution immediately once prepared. If the infusion solution is not administered immediately and needs to be stored, the time from preparation until the completion of the infusion should not exceed:
- 28 days in a refrigerator at 2°C to 8°C (36°F to 46°F)
- 8 hours at room temperature up to 25°C (77°F)
- Do not freeze.
- Do not shake.
Administration
- Administer infusion solution intravenously over 60 minutes through an intravenous line containing a sterile, low-protein binding 0.2 or 0.22 micron in-line filter.
- Use separate infusion bags and filters for each drug product.
IMFINZI in Combination with Other Products
- Administer all intravenous drug products as separate infusions.
- Do not co-administer other intravenous drugs through the same infusion line.
- For platinum-based chemotherapy, refer to Prescribing Information for administration information.
- For pemetrexed therapy, refer to Prescribing Information for administration information.
Combination Regimens: Order of Infusions
IMFINZI in Combination with Tremelimumab-actl
- Infuse tremelimumab-actl first, followed by IMFINZI on the same day of dosing.
IMFINZI in Combination with Tremelimumab-actl and Platinum-Based Chemotherapy
- Infuse tremelimumab-actl first, followed by IMFINZI and then platinum-based chemotherapy on the day of dosing.
IMFINZI in Combination with Tremelimumab-actl and Pemetrexed Therapy
- Infuse tremelimumab-actl first, followed by IMFINZI and then pemetrexed therapy on the day of dosing.
IMFINZI in Combination with Chemotherapy
- Infuse IMFINZI first and then chemotherapy on the same day of dosing.
Combination Regimens: Infusion Instructions
IMFINZI in Combination with Tremelimumab-actl
- Administer tremelimumab-actl over 60 minutes followed by a 60 minute observation period. Then administer IMFINZI as a separate intravenous infusion over 60 minutes.
IMFINZI in Combination with Tremelimumab-actl and Platinum-Based Chemotherapy/ Pemetrexed Therapy
Cycle 1
- Infuse tremelimumab-actl over 60 minutes. One to two hours after completion of tremelimumab-actl infusion, infuse IMFINZI over 60 minutes. One to two hours after completion of IMFINZI infusion, administer platinum-based chemotherapy.
Subsequent Cycles
- If there are no infusion reactions during cycle 1, subsequent cycles of IMFINZI can be given immediately after tremelimumab-actl. The time between the end of the IMFINZI infusion and the start of chemotherapy can be reduced to 30 minutes.
DOSAGE FORMS AND STRENGTHS
Injection: 120 mg/2.4 mL (50 mg/mL) and 500 mg/10 mL (50 mg/mL) clear to opalescent, colorless to slightly yellow solution in a single-dose vial.
USE IN SPECIFIC POPULATIONS
Lactation: Advise not to breastfeed. (8.2 )
Pregnancy
Risk Summary
Based on findings from animal studies and its mechanism of action, IMFINZI can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1) ] . There are no available data on the use of IMFINZI in pregnant women.
In animal reproduction studies, administration of durvalumab to pregnant cynomolgus monkeys from the confirmation of pregnancy through delivery at exposure levels approximately 6 to 20 times higher than those observed at the clinical dose of 10 mg/kg based on area under the curve (AUC), resulted in an increase in premature delivery, fetal loss, and premature neonatal death ( see Data ). Human immunoglobulin G1 (IgG1) is known to cross the placental barrier; therefore, durvalumab has the potential to be transmitted from the mother to the developing fetus. Apprise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
As reported in the literature, the PD-1/PD-L1 pathway plays a central role in preserving pregnancy by maintaining maternal immune tolerance to the fetus. In mouse allogeneic pregnancy models, disruption of PD-L1 signaling was shown to result in an increase in fetal loss. The effects of durvalumab on prenatal and postnatal development were evaluated in reproduction studies in cynomolgus monkeys. Durvalumab was administered from the confirmation of pregnancy through delivery at exposure levels approximately 6 to 20 times higher than those observed at a clinical dose of 10 mg/kg (based on AUC). Administration of durvalumab resulted in premature delivery, fetal loss (abortion and stillbirth), and increase in neonatal deaths. Durvalumab was detected in infant serum on postpartum Day 1, indicating the presence of placental transfer of durvalumab. Based on its mechanism of action, fetal exposure to durvalumab may increase the risk of developing immune-mediated disorders or altering the normal immune response and immune-mediated disorders have been reported in PD-1 knockout mice.
Lactation
Risk Summary
There are no data on the presence of durvalumab in human milk, its effects on the breastfed child, or the effects on milk production. Maternal IgG is known to be present in human milk. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed child to IMFINZI are unknown. Durvalumab was present in the milk of lactating cynomolgus monkeys and was associated with premature neonatal death (see Data).
Because of the potential for adverse reactions in a breastfed child, advise women not to breastfeed during treatment with IMFINZI and for 3 months after the last dose. Refer to the Prescribing Information for the agents administered in combination with IMFINZI for recommended duration to not breastfeed, as appropriate.
Data
In lactating cynomolgus monkeys, durvalumab was present in breast milk at about 0.15% of maternal serum concentrations after administration of durvalumab from the confirmation of pregnancy through delivery at exposure levels approximately 6 to 20 times higher than those observed at the recommended clinical dose of 10 mg/kg (based on AUC). Administration of durvalumab resulted in premature neonatal death .
Females and Males of Reproductive Potential
Pregnancy testing
Verify pregnancy status of females of reproductive potential prior to initiating treatment with IMFINZI.
Contraception
Females
IMFINZI can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1) ] . Advise females of reproductive potential to use effective contraception during treatment with IMFINZI and for 3 months following the last dose of IMFINZI. Refer to the Prescribing Information for the agents administered in combination with IMFINZI for recommended contraception duration, as appropriate.
Pediatric Use
The safety and effectiveness of IMFINZI have not been established in pediatric patients. Safety and efficacy were assessed but not established in a multi-center, open-label study (NCT03837899) in 45 pediatric patients aged 1 to < 17 years with advanced solid tumors. All 45 patients received at least a single dose of IMFINZI, and 41 patients received IMFINZI in combination with tremelimumab-actl. No new safety signals were observed in pediatric patients in this study.
Durvalumab systemic exposure in pediatric patients weighing ≥ 35 kg was within the range of values previously observed in adults given the same weight-based dose, whereas the systemic exposure in pediatric patients weighing < 35 kg was lower than that observed in adults.
Geriatric Use
Of the 401 patients with resectable NSCLC treated with IMFINZI in combination with chemotherapy in the AEGEAN study, 209 (52%) patients were 65 years or older and 49 (12%) patients were 75 years or older. There were no overall clinically meaningful differences in safety or efficacy between patients ≥ 65 years of age and younger patients.
Of the 476 patients with unresectable, Stage III NSCLC treated with IMFINZI in the PACIFIC study, 45% were 65 years or older, while 7.6% were 75 years or older. No overall differences in safety or effectiveness were observed between patients 65 years or older and younger patients. The PACIFIC study did not include sufficient numbers of patients aged 75 years and over to determine whether they respond differently from younger patients.
Of the 330 patients with metastatic NSCLC treated with IMFINZI in combination with tremelimumab-actl and platinum-based chemotherapy, 143 (43%) patients were 65 years or older and 35 (11%) patients were 75 years or older. There were no clinically meaningful differences in safety or efficacy between patients 65 years or older and younger patients.
Of the 262 patients with LS-SCLC treated with IMFINZI, 103 (39%) patients were 65 years or older and 15 (6%) patients were 75 years or older. There were no clinically meaningful differences in safety and efficacy between patients 65 years or older and younger patients.
Of the 265 patients with ES-SCLC treated with IMFINZI in combination with chemotherapy 101 (38%) patients were 65 years or older and 19 (7.2%) patients were 75 years or older. There were no clinically meaningful differences in safety or efficacy between patients 65 years or older and younger patients.
Of the 338 patients with BTC treated with IMFINZI in combination with chemotherapy in the TOPAZ-1 study, 158 (47%) patients were 65 years or older and 38 (11%) patients were 75 years or older. No overall differences in safety or effectiveness of IMFINZI have been observed between patients 65 years of age and older and younger adult patients.
Of the 393 patients with uHCC treated with IMFINZI in combination with tremelimumab-actl, 50% of patients were 65 years of age or older and 13% of patients were 75 years of age or older. No overall differences in safety or effectiveness of IMFINZI have been observed between patients 65 years of age and older and younger adult patients.
Of the 235 patients with endometrial cancer treated with IMFINZI with carboplatin and paclitaxel, 49% of patients were 65 years of age or older and 12% of patients were 75 years of age or older. No overall differences in safety or effectiveness of IMFINZI have been observed between patients 65 years of age and older and younger adult patients.
Of the 530 patients with MIBC treated with IMFINZI in combination with gemcitabine and cisplatin in the NIAGARA study, 272 (51%) patients were 65 years or older, and 57 (11%) patients were 75 years or older. No overall differences in safety or effectiveness of IMFINZI have been observed between patients 65 years of age and older and younger adult patients.
Of the 475 patients with resectable GC/GEJC treated with IMFINZI in combination with FLOT chemotherapy in the MATTERHORN study, 184 (39%) patients were 65 years or older and 37 (8%) patients were 75 years or older. No overall differences in safety or effectiveness were observed between patients ≥ 65 years of age and younger adult patients.
CONTRAINDICATIONS
None.
WARNINGS AND PRECAUTIONS
- Immune-Mediated Adverse Reactions (5.1 )
- Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue, including the following: immune-mediated pneumonitis, immune-mediated colitis, immune-mediated hepatitis, immune-mediated endocrinopathies, immune-mediated dermatologic adverse reactions, immune-mediated nephritis and renal dysfunction, solid organ transplant rejection, and immune-mediated pancreatitis.
- Monitor for early identification and management. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment.
- Withhold or permanently discontinue based on severity and type of reaction.
- Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue, including the following: immune-mediated pneumonitis, immune-mediated colitis, immune-mediated hepatitis, immune-mediated endocrinopathies, immune-mediated dermatologic adverse reactions, immune-mediated nephritis and renal dysfunction, solid organ transplant rejection, and immune-mediated pancreatitis.
- Infusion-Related Reactions: Interrupt, slow the rate of infusion, or permanently discontinue IMFINZI based on the severity of the reaction. (5.2 )
- Complications of Allogeneic HSCT: Fatal and other serious complications can occur in patients who receive allogeneic HSCT before or after being treated with a PD-1/PD-L1 blocking antibody. (5.3)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and use of effective contraception. (5.4 , 8.1 , 8.3 )
Immune-Mediated Adverse Reactions
IMFINZI is a monoclonal antibody that belongs to a class of drugs that bind to either the programmed death-receptor 1 (PD-1) or the PD-ligand 1 (PD-L1), blocking the PD-1/PD-L1 pathway, thereby removing inhibition of the immune response, potentially breaking peripheral tolerance and inducing immune-mediated adverse reactions. Important immune-mediated adverse reactions listed under Warnings and Precautions may not include all possible severe and fatal immune-mediated reactions.
The incidence and severity of immune-mediated adverse reactions were similar when IMFINZI was administered as a single agent or in combination with chemotherapy or in combination with tremelimumab-actl and platinum-based chemotherapy, unless otherwise noted.
Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. Immune-mediated adverse reactions can occur at any time after starting treatment with a PD 1/PD L1 blocking antibody. While immune-mediated adverse reactions usually manifest during treatment with PD-1/PD-L1 blocking antibodies, immune-mediated adverse reactions can also manifest after discontinuation of PD-1/PD-L1 blocking antibodies.
Early identification and management of immune-mediated adverse reactions are essential to ensure safe use of PD-1/PD-L1 blocking antibodies. Monitor patients closely for symptoms and signs that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate liver enzymes, creatinine, and thyroid function at baseline and periodically during treatment. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate.
Withhold or permanently discontinue IMFINZI depending on severity [see Dosage and Administration (2.3) ] . In general, if IMFINZI requires interruption or discontinuation, administer systemic corticosteroid therapy (1 mg to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy.
Toxicity management guidelines for adverse reactions that do not necessarily require systemic steroids (e.g., endocrinopathies and dermatologic reactions) are discussed below.
Immune-Mediated Pneumonitis
IMFINZI can cause immune-mediated pneumonitis. The incidence of pneumonitis is higher in patients who have received prior thoracic radiation.
IMFINZI as a Single Agent
In Patients Who Did Not Receive Recent Prior Radiation
In patients who received IMFINZI on clinical studies in which radiation therapy was generally not administered immediately prior to initiation of IMFINZI, the incidence of immune-mediated pneumonitis was 2.4% (34/1414), including fatal (< 0.1%), and Grade 3-4 (0.4%) adverse reactions. Events resolved in 19 of the 34 patients and resulted in permanent discontinuation in 5 patients. Systemic corticosteroids were required in 19 patients (19/34) with pneumonitis who did not receive chemoradiation prior to initiation of IMFINZI.
The frequency and severity of immune-mediated pneumonitis in patients who did not receive definitive chemoradiation prior to IMFINZI were similar whether IMFINZI was given as a single agent in patients with various cancers in a pooled data set or in patients with ES-SCLC or BTC when given in combination with chemotherapy.
In Patients Who Received Recent Prior Radiation
The incidence of pneumonitis (including radiation pneumonitis) in patients with unresectable Stage III NSCLC following definitive chemoradiation within 42 days prior to initiation of IMFINZI in PACIFIC was 18.3% (87/475) in patients receiving IMFINZI and 12.8% (30/234) in patients receiving placebo. Of the patients who received IMFINZI (475), 1.1% had a fatal adverse reaction and 2.7% had Grade 3 adverse reactions. Events resolved in 50 of the 87 (57%) patients and resulted in permanent discontinuation in 27 of the 87 (31%) patients. Systemic corticosteroids were required in 64 patients (64/87) with pneumonitis who had received chemoradiation prior to initiation of IMFINZI, while 2 patients required use of infliximab with high-dose steroids.
The incidence of pneumonitis (including radiation pneumonitis) in patients with LS-SCLC following chemoradiation within 42 days prior to initiation of IMFINZI in ADRIATIC was 14% (37/262) in patients receiving IMFINZI and 6% (16/265) in patients receiving placebo. Of the patients who received IMFINZI (262), 0.4% had a fatal adverse reaction and 2.7% had Grade 3 adverse reactions. Events resolved in 19 of the 37 (51%) patients and resulted in permanent discontinuation in 18 of the 37 (49%) patients. Systemic corticosteroids were required in all patients, while 1 patient required use of infliximab with high-dose steroids .
IMFINZI with Tremelimumab-actl
Immune-mediated pneumonitis occurred in 1.3% (5/388) of patients receiving IMFINZI in combination with tremelimumab-actl, including fatal (0.3%) and Grade 3 (0.2%) adverse reactions. Events resolved in 3 of the 5 patients and resulted in permanent discontinuation in 1 patient. Systemic corticosteroids were required in all patients; of these, 4 patients required high-dose corticosteroid treatment (at least 40 mg prednisone or equivalent per day). One patient (1/5) required other immunosuppressants.
IMFINZI with Tremelimumab-actl and Platinum-Based Chemotherapy
Immune-mediated pneumonitis occurred in 3.5% (21/596) of patients receiving IMFINZI in combination with tremelimumab-actl and platinum-based chemotherapy, including fatal (0.5%), and Grade 3 (1%) adverse reactions. Events resolved in 11 of the 21 patients and resulted in permanent discontinuation in 7 patients. Systemic corticosteroids were required in all patients with immune-mediated pneumonitis, while 1 patient (1/21) required other immunosuppressants.
Immune-Mediated Colitis
IMFINZI can cause immune-mediated colitis that is frequently associated with diarrhea. Cytomegalovirus (CMV) infection/reactivation has been reported in patients with corticosteroid-refractory immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup to exclude alternative etiologies.
IMFINZI as a Single Agent
Immune-mediated colitis occurred in 2% (37/1889) of patients receiving IMFINZI, including Grade 4 (< 0.1%) and Grade 3 (0.4%) adverse reactions. Events resolved in 27 of the 37 patients and resulted in permanent discontinuation in 8 patients. Systemic corticosteroids were required in all patients with immune-mediated colitis, while 2 patients (2/37) required other immunosuppressants (e.g., infliximab, mycophenolate).
IMFINZI with Tremelimumab-actl
Immune-mediated colitis or diarrhea occurred in 6% (23/388) of patients receiving IMFINZI in combination with tremelimumab-actl, including Grade 3 (3.6%) adverse reactions. Events resolved in 22 of the 23 patients and resulted in permanent discontinuation in 5 patients. All patients received systemic corticosteroids, and 20 of the 23 patients received high-dose corticosteroid treatment (at least 40 mg prednisone or equivalent per day). Three patients also received other immunosuppressants.
Intestinal perforation has been observed in other studies of IMFINZI in combination with tremelimumab-actl.
IMFINZI with Tremelimumab-actl and Platinum-Based Chemotherapy
Immune-mediated colitis occurred in 6.5% (39/596) of patients receiving IMFINZI in combination with tremelimumab-actl including fatal (0.2%) and Grade 3 (2.5%) adverse reactions. Events resolved in 33 of 39 patients and resulted in permanent discontinuation in 11 patients. Systemic corticosteroids were required in all patients with immune-mediated colitis, while 4 patients (4/39) required other corticosteroids.
Intestinal perforation and large intestine perforation were reported in 0.1% of patients receiving IMFINZI in combination with tremelimumab-actl.
Immune-Mediated Hepatitis
IMFINZI can cause immune-mediated hepatitis.
IMFINZI as a Single Agent
Immune-mediated hepatitis occurred in 2.8% (52/1889) of patients receiving IMFINZI, including fatal (0.2%), Grade 4 (0.3%) and Grade 3 (1.4%) adverse reactions. Events resolved in 21 of the 52 patients and resulted in permanent discontinuation of IMFINZI in 6 patients. Systemic corticosteroids were required in all patients with immune-mediated hepatitis, while 2 patients (2/52) required use of mycophenolate with high-dose steroids.
IMFINZI with Tremelimumab-actl
Immune-mediated hepatitis occurred in 7.5% (29/388) of patients receiving IMFINZI in combination with tremelimumab-actl, including fatal (0.8%), Grade 4 (0.3%), and Grade 3 (4.1%) adverse reactions. Events resolved in 12 of the 29 patients and resulted in permanent discontinuation in 9 patients. Systemic corticosteroids were required in all 29 patients and all 29 patients required high-dose corticosteroid treatment (at least 40 mg prednisone or equivalent per day). Eight patients (8/29) required other immunosuppressants.
IMFINZI with Tremelimumab-actl and Platinum-Based Chemotherapy
Immune-mediated hepatitis occurred in 3.9% (23/596) of patients receiving IMFINZI in combination with tremelimumab-actl, including fatal (0.3%), Grade 4 (0.5%), and Grade 3 (2.0%) adverse reactions. Events resolved in 12 of the 23 patients and resulted in permanent discontinuation in 10 patients. Systemic corticosteroids were required in all patients with immune-mediated hepatitis, while 2 patients (2/23) required use of other immunosuppressants.
Immune-Mediated Endocrinopathies
Adrenal Insufficiency
IMFINZI can cause primary or secondary adrenal insufficiency. For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment, including hormone replacement as clinically indicated. Withhold or permanently discontinue IMFINZI based on the severity [see Dosage and Administration (2.3) ] .
IMFINZI as a Single Agent
Immune-mediated adrenal insufficiency occurred in 0.5% (9/1889) of patients receiving IMFINZI, including Grade 3 (< 0.1%) adverse reactions. Events resolved in 1 of the 9 patients and did not lead to permanent discontinuation of IMFINZI in any patients. Systemic corticosteroids were required in all patients with adrenal insufficiency; of these, the majority remained on systemic corticosteroids.
IMFINZI with Tremelimumab-actl
Immune-mediated adrenal insufficiency occurred in 1.5% (6/388) of patients receiving IMFINZI in combination with tremelimumab-actl, including Grade 3 (0.3%) adverse reactions. Events resolved in 2 of the 6 patients. Systemic corticosteroids were required in all 6 patients, and of these, 1 patient required high-dose corticosteroid treatment (at least 40 mg prednisone or equivalent per day).
IMFINZI with Tremelimumab-actl and Platinum-Based Chemotherapy
Immune-mediated adrenal insufficiency occurred in 2.2% (13/596) of patients receiving IMFINZI in combination with tremelimumab-actl, including Grade 3 (0.8%) adverse reactions. Events resolved in 2 of the 13 patients and resulted in permanent discontinuation in 1 patient. Systemic corticosteroids were required in all patients with adrenal insufficiency. One patient also required endocrine therapy.
Hypophysitis
IMFINZI can cause immune-mediated hypophysitis. Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual field cuts. Hypophysitis can cause hypopituitarism. Initiate symptomatic treatment including hormone replacement as clinically indicated. Withhold or permanently discontinue IMFINZI depending on severity [see Dosage and Administration (2.3) ] .
IMFINZI as a Single Agent
Grade 3 hypophysitis/hypopituitarism occurred in < 0.1% (1/1889) of patients who received IMFINZI. Treatment with systemic corticosteroids was administered in this patient. The event did not lead to permanent discontinuation of IMFINZI.
IMFINZI with Tremelimumab-actl
Immune-mediated hypophysitis/hypopituitarism occurred in 1% (4/388) of patients receiving IMFINZI in combination with tremelimumab-actl. Events resolved in 2 of the 4 patients. Systemic corticosteroids were required in 3 patients, and of these, 1 patient received high-dose corticosteroid treatment (at least 40 mg prednisone or equivalent per day). Two patients also required endocrine therapy.
IMFINZI with Tremelimumab-actl and Platinum-Based Chemotherapy
Immune-mediated hypophysitis occurred in 1.3% (8/596) of patients receiving IMFINZI in combination with tremelimumab-actl, including Grade 3 (0.5%) adverse reactions. Events resulted in permanent discontinuation in 1 patient. Systemic corticosteroids were required in 6 patients with immune-mediated hypophysitis; of these, 2 of the 8 patients received high-dose corticosteroid treatment (at least 40 mg prednisone or equivalent per day). Four patients also required endocrine therapy.
Thyroid Disorders
IMFINZI can cause immune-mediated thyroid disorders. Thyroiditis can present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism. Initiate hormone replacement therapy for hypothyroidism or institute medical management of hyperthyroidism as clinically indicated. Withhold or discontinue IMFINZI based on the severity [see Dosage and Administration (2.3) ] .
Thyroiditis
IMFINZI as a Single Agent
Immune-mediated thyroiditis occurred in 0.5% (9/1889) of patients receiving IMFINZI, including Grade 3 (< 0.1%) adverse reactions. Events resolved in 4 of the 9 patients and resulted in permanent discontinuation in 1 patient. Systemic corticosteroids were required in 3 patients (3/9) with immune-mediated thyroiditis, while 8 patients (8/9) required endocrine therapy.
IMFINZI with Tremelimumab-actl
Immune-mediated thyroiditis occurred in 1.5% (6/388) of patients receiving IMFINZI in combination with tremelimumab-actl. Events resolved in 2 of the 6 patients. Systemic corticosteroids were required in 2 patients (2/6) with immune-mediated thyroiditis; of these, 1 patient required high-dose corticosteroid treatment (at least 40 mg prednisone or equivalent per day). All patients required other therapy including hormone replacement therapy, thiamazole, carbimazole, propylthiouracil, perchlorate, calcium channel blocker, or beta-blocker.
IMFINZI with Tremelimumab-actl and Platinum-Based Chemotherapy
Immune-mediated thyroiditis occurred in 1.2% (7/596) of patients receiving IMFINZI in combination with tremelimumab-actl. Events resolved in 2 of the 7 patients and one resulted in permanent discontinuation. Systemic corticosteroids were required in 2 patients (2/7) with immune-mediated thyroiditis, while all patients required endocrine therapy.
Hyperthyroidism
IMFINZI as a Single Agent
Immune-mediated hyperthyroidism occurred in 2.1% (39/1889) of patients receiving IMFINZI. Events resolved in 30 of the 39 patients and did not lead to permanent discontinuation of IMFINZI in any patients. Systemic corticosteroids were required in 9 patients (9/39) with immune-mediated hyperthyroidism, while 35 patients (35/39) required endocrine therapy.
IMFINZI with Tremelimumab-actl
Immune-mediated hyperthyroidism occurred in 4.6% (18/388) of patients receiving IMFINZI in combination with tremelimumab-actl, including Grade 3 (0.3%) adverse reactions. Events resolved in 15 of the 18 patients. Two patients (2/18) required high-dose corticosteroid treatment (at least 40 mg prednisone or equivalent per day). Seventeen patients required other therapy (thiamazole, carbimazole, propylthiouracil, perchlorate, calcium channel blocker, or beta-blocker).
IMFINZI with Tremelimumab-actl and Platinum-Based Chemotherapy
Immune-mediated hyperthyroidism occurred in 5% (30/596) of patients receiving IMFINZI in combination with tremelimumab-actl, including Grade 3 (0.2%) adverse reactions. Events resolved in 21 of the 30 patients. Systemic corticosteroids were required in 5 patients (5/30) with immune-mediated hyperthyroidism, while 28 patients (28/30) required endocrine therapy.
Hypothyroidism
IMFINZI as a Single Agent
Immune-mediated hypothyroidism occurred in 8.3% (156/1889) of patients receiving IMFINZI, including Grade 3 (<0.1%) adverse reactions. Events resolved in 31 of the 156 patients and did not lead to permanent discontinuation of IMFINZI in any patients. Systemic corticosteroids were required in 11 patients (11/156) and the majority of patients (152/156) required long-term thyroid hormone replacement.
IMFINZI with Tremelimumab-actl
Immune-mediated hypothyroidism occurred in 11% (42/388) of patients receiving IMFINZI in combination with tremelimumab-actl. Events resolved in 5 of the 42 patients. One patient received high-dose corticosteroid treatment (at least 40 mg prednisone or equivalent per day). All patients required other therapy (thiamazole, carbimazole, propylthiouracil, perchlorate, calcium channel blocker, or beta-blocker).
IMFINZI with Tremelimumab-actl and Platinum-Based Chemotherapy
Immune-mediated hypothyroidism occurred in 8.6% (51/596) of patients receiving IMFINZI in combination with tremelimumab-actl, including Grade 3 (0.5%) adverse reactions. Systemic corticosteroids were required in 2 patients (2/51) and all patients required endocrine therapy.
IMFINZI with Carboplatin and Paclitaxel
Immune-mediated hypothyroidism occurred in 14% (34/235) of patients receiving IMFINZI in combination with carboplatin and paclitaxel. Events resolved in 8 of the 34 patients. Endocrine therapy was required in 34 of the 34 patients.
Type 1 Diabetes Mellitus, which can present with diabetic ketoacidosis
Monitor patients for hyperglycemia or other signs and symptoms of diabetes. Initiate treatment with insulin as clinically indicated. Withhold or permanently discontinue IMFINZI based on the severity [see Dosage and Administration (2.3) ].
IMFINZI as a Single Agent
Grade 3 immune-mediated type 1 diabetes mellitus occurred in < 0.1% (1/1889) of patients receiving IMFINZI. This patient required long-term insulin therapy and IMFINZI was permanently discontinued. Two additional patients (0.1%, 2/1889) had events of hyperglycemia requiring insulin therapy that did not resolve at the time of reporting.
IMFINZI with Tremelimumab-actl
Two patients (0.5%, 2/388) had events of hyperglycemia requiring insulin therapy that had not resolved at last follow-up.
IMFINZI with Tremelimumab-actl and Platinum-Based Chemotherapy
Immune-mediated Type 1 diabetes mellitus occurred in 0.5% (3/596) of patients receiving IMFINZI in combination with tremelimumab-actl, including Grade 3 (0.3%) adverse reactions. All patients required endocrine therapy.
Immune-Mediated Nephritis with Renal Dysfunction
IMFINZI can cause immune-mediated nephritis.
IMFINZI as a Single Agent
Immune-mediated nephritis occurred in 0.5% (10/1889) of patients receiving IMFINZI, including Grade 3 (< 0.1%) adverse reactions. Events resolved in 5 of the 10 patients and resulted in permanent discontinuation in 3 patients. Systemic corticosteroids were required in all patients with immune-mediated nephritis.
IMFINZI with Tremelimumab-actl
Immune-mediated nephritis occurred in 1% (4/388) of patients receiving IMFINZI in combination with tremelimumab-actl, including Grade 3 (0.5%) adverse reactions. Events resolved in 3 of the 4 patients and resulted in permanent discontinuation in 2 patients. Systemic corticosteroids were required in all patients with immune-mediated nephritis; of these, 3 patients required high-dose corticosteroid treatment (at least 40 mg prednisone or equivalent per day).
IMFINZI with Tremelimumab-actl and Platinum-Based Chemotherapy
Immune-mediated nephritis occurred in 0.7% (4/596) of patients receiving IMFINZI in combination with tremelimumab-actl, including Grade 3 (0.2%) adverse reactions. Events resolved in 1 of the 4 patients and resulted in permanent discontinuation in 3 patients. Systemic corticosteroids were required in all patients with immune-mediated nephritis.
Immune-Mediated Dermatology Reactions
IMFINZI can cause immune-mediated rash or dermatitis. Exfoliative dermatitis, including Stevens Johnson Syndrome (SJS), drug rash with eosinophilia and systemic symptoms (DRESS), and toxic epidermal necrolysis (TEN), has occurred with PD-1/L-1 blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-exfoliative rashes. Withhold or permanently discontinue IMFINZI depending on severity [see Dosage and Administration (2.3) ] .
IMFINZI as a Single Agent
Immune-mediated rash or dermatitis occurred in 1.8% (34/1889) of patients receiving IMFINZI, including Grade 3 (0.4%) adverse reactions. Events resolved in 19 of the 34 patients and resulted in permanent discontinuation in 2 patients. Systemic corticosteroids were required in all patients with immune-mediated rash or dermatitis.
IMFINZI with Tremelimumab-actl
Immune-mediated rash or dermatitis occurred in 4.9% (19/388) of patients receiving IMFINZI in combination with tremelimumab-actl, including Grade 4 (0.3%) and Grade 3 (1.5%) adverse reactions. Events resolved in 13 of the 19 patients and resulted in permanent discontinuation in 2 patients. Systemic corticosteroids were required in all patients with immune-mediated rash or dermatitis; of these, 12 patients required high-dose corticosteroid treatment (at least 40 mg prednisone or equivalent per day). One patient received other immunosuppressants.
IMFINZI with Tremelimumab-actl and Platinum-Based Chemotherapy
Immune-mediated rash or dermatitis occurred in 7.2% (43/596) of patients receiving IMFINZI in combination with tremelimumab-actl, including Grade 3 (0.3%) adverse reactions. Events resolved in 32 of the 43 patients and resulted in permanent discontinuation in 2 patients. Systemic corticosteroids were required in all patients with immune-mediated rash or dermatitis.
Immune-Mediated Pancreatitis
IMFINZI in combination with tremelimumab-actl can cause immune-mediated pancreatitis.
IMFINZI with Tremelimumab-actl
Immune-mediated pancreatitis occurred in 2.3% (9/388) of patients receiving IMFINZI in combination with tremelimumab-actl, including Grade 4 (0.3%) and Grade 3 (1.5%) adverse reactions. Events resolved in 6 of the 9 patients. Systemic corticosteroids were required in all 9 patients, and of these 7 patients required high-dose corticosteroid treatment (at least 40 mg prednisone or equivalent per day).
Other Immune-Mediated Adverse Reactions
The following clinically significant, immune-mediated adverse reactions occurred at an incidence of less than 1% each in patients who received IMFINZI or IMFINZI in combination with tremelimumab-actl, or were reported with the use of other PD-1/PD-L1 blocking antibodies.
Cardiac/vascular: Myocarditis, pericarditis, vasculitis.
Nervous system: Meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis, autoimmune neuropathy.
Ocular: Uveitis, iritis, and other ocular inflammatory toxicities can occur. Some cases can be associated with retinal detachment. Various grades of visual impairment to include blindness can occur. If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Vogt-Koyanagi-Harada-like syndrome, as this may require treatment with systemic steroids to reduce the risk of permanent vision loss.
Gastrointestinal: Pancreatitis including increases in serum amylase and lipase levels, gastritis, duodenitis.
Musculoskeletal and connective tissue disorders: Myositis/polymyositis, rhabdomyolysis and associated sequelae including renal failure, arthritis, polymyalgia rheumatic.
Endocrine: Hypoparathyroidism.
Other (hematologic/immune): Hemolytic anemia, aplastic anemia, hemophagocytic lymphohistiocytosis, systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis), sarcoidosis, immune thrombocytopenia, solid organ transplant rejection, other transplant (including corneal graft) rejection.
Infusion-Related Reactions
IMFINZI can cause severe or life-threatening infusion-related reactions.
Monitor for signs and symptoms of infusion-related reactions. Interrupt, slow the rate of, or permanently discontinue IMFINZI based on the severity [see Dosage and Administration (2.3) ] . For Grade 1 or 2 infusion-related reactions, consider using pre-medications with subsequent doses.
IMFINZI as a Single Agent
Infusion-related reactions occurred in 2.2% (42/1889) of patients receiving IMFINZI, including Grade 3 (0.3%) adverse reactions.
IMFINZI in Combination with Tremelimumab-actl
Infusion-related reactions occurred in 2.6% (10/388) of patients receiving IMFINZI in combination with tremelimumab-actl.
IMFINZI with Tremelimumab-actl and Platinum-Based Chemotherapy
Infusion-related reactions occurred in 2.9% (17/596) of patients receiving IMFINZI in combination with tremelimumab-actl, including Grade 3 (0.3%) adverse reactions.
Complications of Allogeneic HSCT after IMFINZI
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with a PD-1/L-1 blocking antibody. Transplant-related complications include hyperacute graft-versus-host-disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between PD-1/L-1 blockade and allogeneic HSCT.
Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with a PD-1/L-1 blocking antibody prior to or after an allogeneic HSCT.
Embryo-Fetal Toxicity
Based on its mechanism of action and data from animal studies, IMFINZI can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, administration of durvalumab to cynomolgus monkeys from the onset of organogenesis through delivery resulted in increased premature delivery, fetal loss and premature neonatal death. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with IMFINZI and for 3 months after the last dose of IMFINZI [see Use in Specific Populations (8.1 , 8.3 )] .
ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling.
- Immune-Mediated Adverse Reactions [see Warnings and Precautions (5.1) ].
- Infusion-Related Reactions [see Warnings and Precautions (5.2) ].
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described in the WARNINGS AND PRECAUTIONS section reflect exposure to IMFINZI as a single agent in a total of 1,889 patients enrolled in the PACIFIC study (a randomized, placebo-controlled study that enrolled 475 patients with unresectable Stage III NSCLC), Study 1108 (an open-label, single-arm, multicohort study that enrolled 970 patients with advanced solid tumors), and an additional open-label, single-arm study (ATLANTIC Study) that enrolled 444 patients with advanced solid tumors, including NSCLC. In these studies, IMFINZI was administered at a dose of 10 mg/kg every 2 weeks. Among the 1889 patients, 38% were exposed for 6 months or more and 18% were exposed for 12 months or more. The data also reflect exposure to IMFINZI 1,500 mg every 4 weeks as a single agent in 262 patients from the ADRIATIC study (a randomized, double-blind study in patients with LS-SCLC) and to IMFINZI in combination with chemotherapy in 265 patients from the CASPIAN study (a randomized, open-label study in patients with ES-SCLC) and in 338 patients from the TOPAZ-1 study (a randomized, double-blind study in patients with BTC). In the CASPIAN and TOPAZ-1 studies, IMFINZI was administered at a dose of 1,500 mg every 3 or 4 weeks.
The data also reflect exposure to IMFINZI 1,120 mg in combination with carboplatin and paclitaxel (every 3 weeks for up to 6 cycles) followed by IMFINZI 1,500 mg (every 4 weeks) as a single agent in 235 patients in DUO-E (a randomized, placebo-controlled trial in endometrial cancer). Among the 235 patients, 77% (181 patients) were exposed to IMFINZI for 6 months or more and 41% (96 patients) for 12 months or more.
The data also reflect exposure to IMFINZI 1,500 mg in combination with tremelimumab-actl 300 mg in 388 patients in HIMALAYA. In the HIMALAYA study patients received IMFINZI 1,500 mg in combination with tremelimumab-actl as a single intravenous infusion of 300 mg, followed by IMFINZI 1,500 mg every 4 weeks. The pooled safety population (N = 596) described in the WARNINGS AND PRECAUTIONS section reflect exposure to IMFINZI 1,500 mg in combination with tremelimumab-actl 75 mg and histology-based platinum chemotherapy regimens in 330 patients in POSEIDON [see Clinical Studies (14.1) ] , and 266 patients with ES-SCLC in CASPIAN who received up to four cycles of platinum-etoposide plus IMFINZI 1,500 mg with tremelimumab-actl 75 mg every 3 weeks, followed by IMFINZI 1,500 mg every 4 weeks (an unapproved regimen for extensive stage small cell lung cancer). Among the 596 patients, 55% were exposed to IMFINZI for 6 months or more and 24% were exposed for 12 months or more.
The data described in this section reflect exposure to IMFINZI in patients with unresectable Stage III NSCLC enrolled in the PACIFIC study, in patients with metastatic NSCLC enrolled in the POSEIDON study, in patients with LS-SCLC enrolled in the ADRIATIC study, in patients with ES-SCLC enrolled in the CASPIAN study, in patients with BTC enrolled in the TOPAZ‑1 study, in patients with uHCC included in the HIMALAYA study, in patients with dMMR endometrial cancer enrolled in the DUO-E study, in patients with resectable NSCLC enrolled in the AEGEAN study, in patients with MIBC enrolled in the NIAGARA study and in patients with resectable GC/GEJC enrolled in the MATTERHORN study.
Non-Small Cell Lung Cancer
Neoadjuvant and Adjuvant Treatment of Resectable NSCLC – AEGEAN
The safety of IMFINZI in combination with neoadjuvant platinum-containing chemotherapy followed by surgery, and continued adjuvant treatment with IMFINZI as a single agent after surgery, was investigated in AEGEAN, a randomized, double-blind, placebo-controlled, multicenter study for patients with resectable NSCLC (Stage IIA to select Stage IIIB [AJCC, 8 th edition]); squamous or non-squamous) [see Clinical Studies (14.1) ] .
Safety data are available for the 799 patients who received IMFINZI in combination with chemotherapy (n=401) or placebo in combination with chemotherapy (n=398).
The median duration of exposure to IMFINZI 1,500 mg every 3 weeks in the neoadjuvant phase was 12 weeks (range: 0 to 19 weeks). The median duration of exposure to IMFINZI 1,500 mg every 4 weeks in the adjuvant phase was 37 weeks (range: 4 to 67 weeks). The median age of patients who received IMFINZI was 65 years (range: 30 to 88), 52% age 65 or older, 12% age 75 or older; 65% male; 54% White, 41% Asian, 1% Black, 3% Other races; and 17% Hispanic or Latino.
The most common adverse reactions (occurring in ≥ 20% of patients) were anemia, nausea, constipation, fatigue, musculoskeletal pain, and rash.
Table 5 summarizes the adverse reactions that occurred in (≥ 10%) patients treated with IMFINZI in combination with chemotherapy.
Adverse Reaction | IMFINZI with Chemotherapy N=401 | Placebo with Chemotherapy N=398 | ||
All Grades (%) | Grade 3 or 4 (%) | All Grades (%) | Grade 3 or 4 (%) | |
Gastrointestinal disorders | ||||
Nausea | 25 | 0.2 | 29 | 0.3 |
Constipation | 25 | 0.2 | 21 | 0 |
Diarrhea includes colitis, diarrhea, enteritis, and proctitis. | 14 | 1 | 13 | 1.3 |
Vomiting | 11 | 0.7 | 11 | 1.0 |
General disorders and administration site conditions | ||||
Fatigue includes fatigue and asthenia. | 25 | 0 | 25 | 1.5 |
Skin and subcutaneous tissue disorders | ||||
Rash includes dermatitis, dermatitis acneiform, drug eruption, eczema, eczema asteatotic, erythema, palmar‑erythrodysaesthesia syndrome, pemphigoid, rash, rash erythematous, rash macular, rash maculo-papular, rash papular, rash pruritic, and rash pustular, skin exfoliation, and urticarial dermatitis. | 22 | 0.5 | 14 | 0.3 |
Pruritus | 12 | 0.2 | 6 | 0 |
Musculoskeletal and connective tissue disorders | ||||
Musculoskeletal pain includes arthralgia, arthritis, back pain, bone pain, chest pain, musculoskeletal chest pain, musculoskeletal pain, musculoskeletal discomfort, musculoskeletal stiffness,myalgia, neck pain, non-cardiac chest pain, pain in extremity, and spinal pain. | 24 | 1 | 29 | 0.5 |
Metabolism and nutrition disorders | ||||
Decreased appetite | 18 | 0.2 | 18 | 0.3 |
Nervous system disorders | ||||
Peripheral neuropathy includes dysaesthesia, hypoaesthesia, neuralgia, neuropathy peripheral, paraesthesia, peripheral sensory neuropathy, and polyneuropathy. | 16 | 0.5 | 22 | 0.8 |
Endocrine disorders | ||||
Hypothyroidism includes blood thyroid stimulating hormone increased and hypothyroidism. | 11 | 0 | 3.8 | 0 |
Respiratory, thoracic and mediastinal disorders | ||||
Cough / Productive cough | 11 | 0 | 13 | 0 |
Pneumonia includes lower respiratory tract infection, lung abscess, paracancerous pneumonia, pneumonia, pneumonia aspiration, pneumonia bacterial, pneumonia chlamydial, pneumonia cryptococcal, pneumonia fungal, pneumonia pseudomonal, pneumonia streptococcal, pneumonia viral, and post-procedural pneumonia. , Five Grade 5 events in the IMFINZI arm and four Grade 5 events in the Placebo arm. | 11 | 3.5 | 10 | 3 |
COVID-19 Includes COVID-19 and COVID-19 Pneumonia. Five Grade 5 events in the IMFINZI arm and One Grade 5 event in the placebo arm. | 11 | 0.2 | 9 | 0.8 |
Psychiatric Disorders | ||||
Insomnia | 10 | 0 | 12 | 0 |
Table 6 summarizes the laboratory abnormalities in patients treated with IMFINZI in combination with chemotherapy.
Laboratory Abnormality Graded per NCI CTCAE V5. | IMFINZI with Chemotherapy The denominator used to calculate the rate varied from 349 to 399 based on the number of patients with a baseline value and at least one post-treatment value. | Placebo with Chemotherapy The denominator used to calculate the rate varied from 333 to 398 based on the number of patients with a baseline value and at least one post-treatment value. | ||
All Grades (%) | Grade 3 or 4 (%) | All Grades (%) | Grade 3 or 4 (%) | |
Hematology | ||||
Hemoglobin decreased | 78 | 10 | 75 | 9 |
Leukocytes decreased | 63 | 12 | 64 | 11 |
Neutrophils decreased | 52 | 24 | 56 | 27 |
Platelets decreased | 46 | 7 | 44 | 8 |
Lymphocytes decreased | 41 | 11 | 37 | 9 |
Chemistry | ||||
Calcium corrected, decreased | 51 | 3.3 | 52 | 4.5 |
Alanine aminotransferase increased | 49 | 6 | 42 | 2 |
Aspartate aminotransferase increased | 47 | 3.5 | 37 | 1.8 |
Potassium increased | 33 | 1.5 | 29 | 2 |
Sodium decreased | 35 | 5 | 33 | 6 |
Gamma glutamyl transferase increased | 36 | 4.7 | 35 | 2.1 |
Creatinine increased | 32 | 2.3 | 27 | 3.3 |
Amylase increased | 25 | 4.7 | 24 | 3.6 |
Magnesium decreased | 22 | 2.8 | 20 | 3.6 |
Lipase increased | 23 | 4.9 | 24 | 7 |
Neoadjuvant Phase of AEGEAN
A total of 401 patients received at least 1 dose of IMFINZI in combination with platinum-containing chemotherapy as neoadjuvant treatment and 398 patients received at least 1 dose of placebo in combination with platinum-containing chemotherapy as neoadjuvant treatment.
Serious adverse reactions occurred in 21% of patients who received IMFINZI in combination with platinum-containing chemotherapy as neoadjuvant treatment; the most frequent (≥1%) serious adverse reactions were pneumonia (2.7%), anemia (1.5%), myelosuppression (1.5%), vomiting (1.2%), neutropenia (1%), and acute kidney injury (1%). Fatal adverse reactions occurred in 2% of patients, including death due to COVID-19 pneumonia (0.5%), sepsis (0.5%), myocarditis (0.2%), decreased appetite (0.2%), hemoptysis (0.2%), and death not otherwise specified (0.2%).
Permanent discontinuation of any study drug due to an adverse reaction occurred in 14% of patients who received IMFINZI in combination with platinum-containing chemotherapy as neoadjuvant treatment; the most frequent (>0.5%) adverse reactions that led to permanent discontinuation of any study drug were anemia (1.5%), neutropenia (0.7%), myelosuppression (0.7%), and periphery sensory neuropathy (0.7%). Permanent discontinuation of IMFINZI due to an adverse reaction occurred in 6.7% of patients who received IMFINZI in combination with platinum-containing chemotherapy as neoadjuvant treatment; the most frequent (≥0.5%) adverse reactions that led to permanent discontinuation of IMFINZI were peripheral sensory neuropathy (0.7%) and pneumonitis (0.5%).
Of the 401 IMFINZI-treated patients and 398 placebo-treated patients who received neoadjuvant treatment, 1.7% (n=7) and 1% (n=4), respectively, did not receive surgery due to adverse reactions. Adverse reactions that led to cancellation of surgery in the IMFINZI arm were COVID-19 pneumonia, HIV infection, pneumonitis, prostate cancer, colon cancer, pruritus, and colitis.
Of the 325 IMFINZI-treated patients who received surgery, 4% (n=15) experienced delay of surgery (a surgical delay is defined as on-study surgery occurring more than 40 days after the last dose of study treatment in the neoadjuvant period) due to adverse reactions. Of the 326 placebo-treated patients who received surgery, 4% (n=16) experienced delay of surgery due to adverse reactions.
Of the 325 IMFINZI-treated patients who received surgery, 6.5% (n=21) did not receive adjuvant treatment due to adverse reactions. Of the 326 placebo-treated patients who received surgery, 5.8% (n=19) did not receive adjuvant treatment due to adverse reactions.
Adjuvant Phase of AEGEAN
A total of 265 patients in the IMFINZI arm and 254 patients in the placebo arm received at least 1 dose of adjuvant treatment.
Of the patients who received single agent IMFINZI as adjuvant treatment, 13% experienced serious adverse reactions. The most frequent serious adverse reactions reported in >1% of patients were pneumonia (1.9%), pneumonitis (1.1%), and COVID-19 (1.1%). Four fatal adverse reactions occurred during the adjuvant phase of the study, including COVID-19 pneumonia, pneumonia aspiration, interstitial lung disease and aortic aneurysm. Permanent discontinuation of adjuvant IMFINZI due to an adverse reaction occurred in 8% of patients. The most frequent (≥0.5%) adverse reaction that led to permanent discontinuation of adjuvant IMFINZI was pneumonitis (1.1%) and rash (0.8%).
Unresectable Stage III NSCLC - PACIFIC
The safety of IMFINZI in patients with Stage III NSCLC who completed concurrent platinum-based chemoradiotherapy within 42 days prior to initiation of study drug was evaluated in the PACIFIC study, a multicenter, randomized, double-blind, placebo-controlled study. A total of 475 patients received IMFINZI 10 mg/kg intravenously every 2 weeks. The study excluded patients who had disease progression following chemoradiation, with active or prior autoimmune disease within 2 years of initiation of the study or with medical conditions that required systemic immunosuppression [see Clinical Studies (14.1) ].
The study population characteristics were: median age of 64 years (range: 23 to 90), 45% age 65 years or older, 70% male, 69% White, 27% Asian, 75% former smoker, 16% current smoker, and 51% had WHO performance status (PS) of 1. All patients received definitive radiotherapy as per protocol, of which 92% received a total radiation dose of 54 Gy to 66 Gy. The median duration of exposure to IMFINZI was 10 months (range: 0.2 to 12.6).
IMFINZI was discontinued due to adverse reactions in 15% of patients. The most common adverse reactions leading to IMFINZI discontinuation were pneumonitis or radiation pneumonitis in 6% of patients. Serious adverse reactions occurred in 29% of patients receiving IMFINZI. The most frequent serious adverse reactions reported in at least 2% of patients were pneumonitis or radiation pneumonitis (7%) and pneumonia (6%). Fatal pneumonitis or radiation pneumonitis and fatal pneumonia occurred in < 2% of patients and were similar across arms. The most common adverse reactions (occurring in ≥ 20% of patients) were cough, fatigue, pneumonitis or radiation pneumonitis, upper respiratory tract infections, dyspnea, and rash.
Table 7 summarizes the adverse reactions that occurred in at least 10% of patients treated with IMFINZI.
IMFINZI N = 475 | Placebo N = 234 | |||
Adverse Reaction | All Grades (%) | Grades 3-4 (%) | All Grades (%) | Grades 3-4 (%) |
Respiratory, Thoracic, and Mediastinal Disorders | ||||
Cough/Productive Cough | 40 | 0.6 | 30 | 0.4 |
Pneumonitis Includes acute interstitial pneumonitis, interstitial lung disease, pneumonitis, pulmonary fibrosis. /Radiation Pneumonitis | 34 | 3.4 | 25 | 3 |
Dyspnea Includes dyspnea, and exertional dyspnea. | 25 | 1.5 | 25 | 2.6 |
General Disorders | ||||
Fatigue Includes asthenia and fatigue. | 34 | 0.8 | 32 | 1.3 |
Pyrexia | 15 | 0.2 | 9 | 0 |
Infections | ||||
Upper respiratory tract infections Includes laryngitis, nasopharyngitis, peritonsillar abscess, pharyngitis, rhinitis, sinusitis, tonsillitis, tracheobronchitis, and upper respiratory tract infection. | 26 | 0.4 | 19 | 0 |
Pneumonia Includes lung infection, pneumocystis jirovecii pneumonia, pneumonia, pneumonia adenoviral, pneumonia bacterial, pneumonia cytomegaloviral, pneumonia haemophilus, pneumonia klebsiella, pneumonia necrotizing, pneumonia pneumococcal, and pneumonia streptococcal. | 17 | 7 | 12 | 6 |
Skin and Subcutaneous Tissue Disorders | ||||
Rash Includes rash erythematous, rash generalized, rash macular, rash maculopapular, rash papular, rash pruritic, rash pustular, erythema, eczema, rash, and dermatitis. | 23 | 0.6 | 12 | 0 |
Pruritus Includes pruritus generalized and pruritus. | 12 | 0 | 6 | 0 |
Gastrointestinal Disorders | ||||
Diarrhea | 18 | 0.6 | 19 | 1.3 |
Abdominal pain Includes abdominal pain, abdominal pain lower, abdominal pain upper, and flank pain. | 10 | 0.4 | 6 | 0.4 |
Endocrine Disorders | ||||
Hypothyroidism Includes autoimmune hypothyroidism and hypothyroidism. | 12 | 0.2 | 1.7 | 0 |
Other adverse reactions occurring in less than 10% of patients treated with IMFINZI were dysphonia, dysuria, night sweats, peripheral edema, and increased susceptibility to infections.
Table 8 summarizes the laboratory abnormalities that occurred in at least 20% of patients treated with IMFINZI.
IMFINZI | Placebo | |||
Laboratory Abnormality | All Grades Graded according to NCI CTCAE version 4.0. (%) Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: IMFINZI (range: 464 to 470) and placebo (range: 224 to 228). | Grade 3 or 4 (%) | All Grades (%) | Grade 3 or 4 (%) |
Chemistry | ||||
Hyperglycemia | 52 | 8 | 51 | 8 |
Hypocalcemia | 46 | 0.2 | 41 | 0 |
Increased ALT | 39 | 2.3 | 22 | 0.4 |
Increased AST | 36 | 2.8 | 21 | 0.4 |
Hyponatremia | 33 | 3.6 | 30 | 3.1 |
Hyperkalemia | 32 | 1.1 | 29 | 1.8 |
Increased GGT | 24 | 3.4 | 22 | 1.7 |
Hematology | ||||
Lymphopenia | 43 | 17 | 39 | 18 |
Metastatic NSCLC - POSEIDON
The safety of IMFINZI in combination with tremelimumab-actl and platinum-based chemotherapy in patients with metastatic NSCLC was evaluated in POSEIDON (NCT03164616), a randomized, open-label, multicenter, active-controlled study. A total of 330 patients received IMFINZI 1,500 mg in combination with tremelimumab-actl (≥ 30 kg body weight received 75 mg and < 30 kg body weight received 1 mg/kg) and histology-based platinum chemotherapy regimens [see Clinical Studies (14.1) ] . Of these patients, 66% received the maximum 5 doses of tremelimumab-actl and 79% received at least 4 doses. Treatment was continued with IMFINZI as a single agent (or with IMFINZI and histologically-based pemetrexed for non-squamous patients based on the investigator’s decision) until disease progression or unacceptable toxicity. The study excluded patients with active or prior autoimmune disease or with medical conditions that required systemic corticosteroids or immunosuppressants [see Clinical Studies (14.1) ].
The median age of patients who received IMFINZI in combination with tremelimumab-actl and platinum-based chemotherapy was 63 years (range: 27 to 87); 80% male; 61% White, 29% Asian, 58% former smoker, 25% current smoker, and 68% ECOG performance of 1.
Serious adverse reactions occurred in 44% of patients receiving IMFINZI in combination with tremelimumab-actl and platinum-based chemotherapy. The most frequent serious adverse reactions reported in at least 2% of patients were pneumonia (11%), anemia (5%), diarrhea (2.4%), thrombocytopenia (2.4%), pyrexia (2.4%), and febrile neutropenia (2.1%). Fatal adverse reactions occurred in a total of 4.2% of patients receiving IMFINZI in combination with tremelimumab-actl and platinum-based chemotherapy. These include hepatitis, nephritis, myocarditis, pancreatitis (all in the same patient), death (2 patients), sepsis (2 patients), pneumonitis (2 patients), acute kidney injury (2 patients), febrile neutropenia (1 patient), chronic obstructive pulmonary disease (COPD) (1 patient), dyspnea (1 patient), sudden death (1 patient), and ischemic stroke (1 patient).
Permanent discontinuation of IMFINZI or tremelimumab-actl due to an adverse reaction occurred in 17% of the patients. Adverse reactions which resulted in permanent discontinuation of IMFINZI or tremelimumab-actl in > 2% of patients included pneumonia.
Dosage interruption or delay of IMFINZI and tremelimumab-actl due to an adverse reaction occurred in 41% of patients. Adverse reactions which required dosage interruption or delay of IMFINZI and tremelimumab-actl in > 1% of patients included anemia, leukopenia/white blood cell count decreased, pneumonia, pneumonitis, colitis, diarrhea, hepatitis, rash, asthenia, amylase increased, alanine aminotransferase increased, aspartate aminotransferase increased, lipase increased, neutropenia/ neutrophil count decreased, and thrombocytopenia/platelet count decreased.
The most common adverse reactions (occurring in ≥ 20% of patients) were nausea, fatigue, musculoskeletal pain, decreased appetite, rash, and diarrhea. Grade 3 or 4 laboratory abnormalities (≥ 10%) were neutropenia, anemia, leukopenia, lymphocytopenia, lipase increased, hyponatremia and thrombocytopenia.
Table 9 summarizes the adverse reactions in POSEIDON.
IMFINZI with tremelimumab-actl and platinum-based chemotherapy N = 330 | Platinum-based chemotherapy N = 333 | |||
Adverse Reaction | All Grades (%) | Grade 3 or 4 (%) | All Grades (%) | Grade 3 or 4 (%) |
Gastrointestinal disorders | ||||
Nausea | 42 | 1.8 | 37 | 2.1 |
Diarrhea | 22 | 1.5 | 15 | 1.5 |
Constipation | 19 | 0 | 24 | 0.6 |
Vomiting | 18 | 1.2 | 14 | 1.5 |
Stomatitis Includes mucosal inflammation and stomatitis. | 10 | 0 | 6 | 0.3 |
General disorders and administration site conditions | ||||
Fatigue/Asthenia Includes asthenia and fatigue. | 36 | 5 | 32 | 4.5 |
Pyrexia Includes body temperature increased, hyperpyrexia, hyperthermia, and pyrexia. | 19 | 0 | 8 | 0 |
Edema Includes face edema, localized edema, and edema peripheral. | 10 | 0 | 10 | 0.6 |
Musculoskeletal and connective tissue disorders | ||||
Musculoskeletal Pain Includes arthralgia, arthritis, back pain, bone pain, musculoskeletal chest pain, musculoskeletal pain, myalgia, neck pain, non-cardiac chest pain, and spinal pain. | 29 | 0.6 | 22 | 1.5 |
Metabolism and nutrition disorders | ||||
Decreased appetite | 28 | 1.5 | 25 | 1.2 |
Skin and subcutaneous tissue disorders | ||||
Rash Includes eczema, erythema, dermatitis, drug eruption, erythema multiforme, pemphigoid, rash, rash maculo-papular, rash papular, rash pruritic, and rash pustular. | 27 | 2.4 | 10 | 0.6 |
Pruritus | 11 | 0 | 4.5 | 0 |
Alopecia | 10 | 0 | 6 | 0 |
Infections and Infestations | ||||
Pneumonia Includes lower respiratory tract infection, pneumocystis jirovecii pneumonia, pneumonia, pneumonia aspiration, and pneumonia bacterial. | 17 | 8 | 12 | 4.2 |
Upper respiratory tract infections Includes laryngitis, nasopharyngitis, pharyngitis, rhinitis, sinusitis, tonsillitis, tracheobronchitis and upper respiratory tract infection. | 15 | 0.6 | 9 | 0.9 |
Endocrine disorders | ||||
Hypothyroidism Includes blood thyroid stimulating hormone increased and hypothyroidism. | 13 | 0 | 2.1 | 0 |
Respiratory, thoracic and mediastinal disorders | ||||
Cough/Productive Cough Includes cough and productive cough. | 12 | 0 | 8 | 0.3 |
Nervous system disorders | ||||
Headache Includes headache and migraine. | 11 | 0 | 8 | 0.6 |
Table 10 summarizes the laboratory abnormalities in POSEIDON.
Laboratory Abnormality Graded according to NCI CTCAE version 4.03. | IMFINZI with tremelimumab-actl and platinum-based chemotherapy The denominator used to calculate the rate varied from 45 to 326 based on the number of patients with a baseline value and at least one post-treatment value. | Platinum-based chemotherapy The denominator used to calculate the rate varied from 43 to 323 based on the number of patients with a baseline value and at least one post-treatment value. | ||
All Grades (%) | Grade 3 or 4 (%) | All Grades (%) | Grade 3 or 4 (%) | |
Chemistry | ||||
Blood creatinine increased | 89 | 4 | 83 | 1.9 |
Increased ALT | 64 | 6 | 56 | 4.7 |
Increased AST | 63 | 5 | 55 | 2.2 |
Hypocalcemia | 58 | 0.9 | 49 | 0.9 |
Hyponatremia | 55 | 13 | 50 | 11 |
Hyperkalemia | 49 | 2.2 | 35 | 2.8 |
Hyperglycemia | 42 | 6 | 37 | 3.1 |
Amylase increased | 41 | 9 | 25 | 6 |
Gamma Glutamyl Transferase increased | 38 | 2.2 | 35 | 4.7 |
Lipase increased | 35 | 14 | 25 | 5 |
Increased Alkaline Phosphatase | 33 | 3.4 | 26 | 1.2 |
Albumin decreased | 27 | 1.9 | 18 | 0.9 |
Hypokalemia | 21 | 7 | 17 | 2.8 |
Bilirubinemia | 16 | 0.9 | 8 | 0.3 |
Hypernatremia | 15 | 0 | 14 | 0 |
Hypomagnesemia | 12 | 4 | 23 | 0 |
Hematology | ||||
Anemia | 84 | 24 | 84 | 25 |
Leukopenia | 77 | 21 | 81 | 18 |
Neutropenia | 71 | 37 | 69 | 32 |
Lymphocytopenia | 67 | 20 | 60 | 19 |
Thrombocytopenia | 53 | 11 | 54 | 12 |
Small Cell Lung Cancer
Limited Stage Small Cell Lung Cancer – ADRIATIC
The safety of IMFINZI as a single agent in patients with LS-SCLC without disease progression following completion of concurrent platinum-based chemoradiotherapy (60-66 Gy once daily over 6 weeks or 45 Gy twice daily over 3 weeks) within 42 days prior to initiation of study drug, was evaluated in the ADRIATIC study, a multicenter, randomized, double-blind, placebo-controlled study [see Clinical Studies (14.2) ]. A total of 262 patients received IMFINZI 1,500 mg every 4 weeks until disease progression or unacceptable toxicity or a maximum of 24 months. The study excluded patients with Stage I or II LS-SCLC who were considered medically operable and patients with active or prior autoimmune disease or with medical conditions that required systemic corticosteroids or immunosuppressants.
The study population characteristics were: median age of 62 years (range: 28 to 84); 39% age 65 years or older, 6% age 75 years or older; 69% male; 50% white, 48% Asian, 1.3% other races; 4.2% Hispanic or Latino; 68% former smoker, 22% current smoker; and 51% had WHO performance status of 1. Sixty-seven percent of patients received a total radiation dose of 60 Gy to 66 Gy once daily and 27% of patients received a total radiation dose of 45 Gy twice daily. The median duration of exposure to IMFINZI was 9.2 months (range: 0.92 to 25) in the IMFINZI arm.
Serious adverse reactions occurred in 30% of patients receiving IMFINZI. The most frequent serious adverse reactions reported in ≥ 1% of patients receiving IMFINZI were pneumonitis or radiation pneumonitis (12%), and pneumonia (5%). Fatal adverse reactions occurred in 2.7% of patients who received IMFINZI including pneumonia (1.5%), cardiac failure, encephalopathy and pneumonitis (0.4% each). Permanent discontinuation of IMFINZI due to adverse reactions occurred in 16% of the patients. Adverse reactions which resulted in permanent discontinuation of IMFINZI in ≥ 1% of patients included pneumonitis or radiation pneumonitis (9%) and pneumonia (1.5%). Dosage interruptions of IMFINZI due to an adverse reaction occurred in 35% of patients. Adverse reactions which required dosage interruption in ≥ 5% of patients included pneumonitis or radiation pneumonitis (17%). The most common adverse reactions occurring in ≥ 20% of patients receiving IMFINZI were pneumonitis or radiation pneumonitis (38%), and fatigue (21%).
Table 11 summarizes the adverse reactions that occurred in patients treated with IMFINZI in the ADRIATIC study.
IMFINZI (N=262) | Placebo (N=265) | |||
Adverse Reaction | All Grades (%) | Grade 3 or 4 (%) | All Grades (%) | Grade 3 or 4 (%) |
Respiratory, thoracic and mediastinal disorders | ||||
Pneumonitis or Radiation pneumonitis Includes pneumonitis, immune-mediated lung disease, interstitial lung disease, radiation pneumonitis, and lung radiation fibrosis | 38 | 3.1 | 30 | 2.6 |
Cough/Productive cough | 17 | 0 | 14 | 0 |
Dyspnea Includes dyspnea and exertional dyspnea. | 11 | 0.4 | 7 | 0 |
General disorders | ||||
Fatigue Includes fatigue and asthenia. | 21 | 0.4 | 20 | 2.3 |
Skin and subcutaneous tissue disorders | ||||
Rash Includes dermatitis, acneiform dermatitis, eczema, rash, maculo-papular rash, papular rash, pruritic rash, and skin exfoliation. | 18 | 0.4 | 11 | 0 |
Pruritus | 13 | 0 | 7 | 0 |
Endocrine disorders | ||||
Hypothyroidism Includes hypothyroidism, increased blood thyroid stimulating hormone, and decreased thyroxine free. | 17 | 0 | 4.9 | 0 |
Hyperthyroidism Includes hyperthyroidism, decreased blood thyroid stimulating hormone, increased thyroxine free, increased thyroxine, increased tri-iodothyronine free, and increased tri-iodothyronine. | 12 | 0 | 1.9 | 0 |
Metabolism and nutrition disorders | ||||
Decreased appetite | 17 | 0 | 13 | 0 |
Nervous system disorders | ||||
Dizziness Includes dizziness, postural dizziness, vertigo, and positional vertigo. | 14 | 0 | 9 | 0 |
Infections and infestations | ||||
Pneumonia Includes pneumonia, atypical pneumonia, lower respiratory tract infection, bacterial pneumonia, pneumocystis jirovecii pneumonia, legionella pneumonia, and viral pneumonia. | 13 | 3.1 | 9 | 4.2 |
Gastrointestinal disorders | ||||
Nausea | 13 | 0 | 11 | 0 |
Diarrhea | 11 | 1.9 | 8 | 0 |
Constipation | 10 | 0 | 10 | 0 |
Table 12 summarizes the laboratory abnormalities that occurred in at least 20% of patients treated with IMFINZI.
Laboratory Abnormality Graded according to NCI CTCAE version 4.03, except creatinine increased which is graded according to NCI CTCAE version 5.0. | IMFINZI The denominator used to calculate the rate varied from 63 to 259 based on the number of patients with a baseline value and at least one post-treatment value. | Placebo The denominator used to calculate the rate varied from 65 to 262 based on the number of patients with a baseline value and at least one post-treatment value. | ||
|
| All Grades (%) | Grade 3 or 4 (%) | |
Chemistry | ||||
Hypocalcemia | 43 | 0 | 43 | 0.8 |
Hyperglycemia | 38 | 3.2 | 45 | 1.5 |
ALT increased | 36 | 2.3 | 29 | 2.3 |
AST increased | 33 | 2.3 | 28 | 1.5 |
Gamma Glutamyl Transferase increased | 32 | 7 | 27 | 2.9 |
Hyponatremia | 32 | 5 | 29 | 6.2 |
Hyperkalemia | 23 | 1.2 | 17 | 0.8 |
Creatinine increased | 21 | 0 | 17 | 0.8 |
Hematology | ||||
Lymphocytes decreased | 34 | 10 | 33 | 10 |
Leukocytes decreased | 26 | 0.4 | 33 | 1.1 |
Extensive Stage Small Cell Lung Cancer – CASPIAN
The safety of IMFINZI in combination with etoposide and either carboplatin or cisplatin in previously untreated ES-SCLC was evaluated in CASPIAN, a randomized, open-label, multicenter, active-controlled study. A total of 265 patients received IMFINZI 1,500 mg in combination with chemotherapy every 3 weeks for 4 cycles followed by IMFINZI 1,500 mg every 4 weeks until disease progression or unacceptable toxicity. The study excluded patients with active or prior autoimmune disease or with medical conditions that required systemic corticosteroids or immunosuppressants [see Clinical Studies (14.2) ] . Among 265 patients receiving IMFINZI, 49% were exposed for 6 months or longer and 19% were exposed for 12 months or longer.
Among 266 patients receiving chemotherapy alone, 57% of the patients received 6 cycles of chemotherapy and 8% of the patients received prophylactic cranial irradiation (PCI) after chemotherapy.
IMFINZI was discontinued due to adverse reactions in 7% of the patients receiving IMFINZI plus chemotherapy. These include pneumonitis, hepatotoxicity, neurotoxicity, sepsis, diabetic ketoacidosis and pancytopenia (1 patient each). Serious adverse reactions occurred in 31% of patients receiving IMFINZI plus chemotherapy. The most frequent serious adverse reactions reported in at least 1% of patients were febrile neutropenia (4.5%), pneumonia (2.3%), anemia (1.9%), pancytopenia (1.5%), pneumonitis (1.1%) and COPD (1.1%). Fatal adverse reactions occurred in 4.9% of patients receiving IMFINZI plus chemotherapy. These include pancytopenia, sepsis, septic shock, pulmonary artery thrombosis, pulmonary embolism, and hepatitis (1 patient each) and sudden death (2 patients). The most common adverse reactions (occurring in ≥ 20% of patients) were nausea, fatigue/asthenia and alopecia.
Table 13 summarizes the adverse reactions that occurred in patients treated with IMFINZI plus chemotherapy.
IMFINZI with etoposide and either carboplatin or cisplatin N = 265 | Etoposide and either carboplatin or cisplatin N = 266 | |||
Adverse Reaction | All Grades (%) | Grade 3-4 (%) | All Grades (%) | Grade 3-4 (%) |
Gastrointestinal disorders | ||||
Nausea | 34 | 0.4 | 34 | 1.9 |
Constipation | 17 | 0.8 | 19 | 0 |
Vomiting | 15 | 0 | 17 | 1.1 |
Diarrhea | 10 | 1.1 | 11 | 1.1 |
General disorders and administration site conditions | ||||
Fatigue/Asthenia | 32 | 3.4 | 32 | 2.3 |
Skin and subcutaneous tissue disorders | ||||
Alopecia | 31 | 1.1 | 34 | 0.8 |
Rash Includes rash erythematous, rash generalized, rash macular, rash maculopapular, rash papular, rash pruritic, rash pustular, erythema, eczema, rash and dermatitis. | 11 | 0 | 6 | 0 |
Metabolism and nutrition disorders | ||||
Decreased appetite | 18 | 0.8 | 17 | 0.8 |
Respiratory, thoracic and mediastinal disorders | ||||
Cough/Productive Cough | 15 | 0.8 | 9 | 0 |
Endocrine disorders | ||||
Hyperthyroidism Includes hyperthyroidism and Basedow's disease. | 10 | 0 | 0.4 | 0 |
Table 14 summarizes the laboratory abnormalities that occurred in at least 20% of patients treated with IMFINZI plus chemotherapy.
IMFINZI with Etoposide and either Carboplatin or Cisplatin | Etoposide and either Carboplatin or Cisplatin | |
Laboratory Abnormality | Grade Graded according to NCI CTCAE version 4.03. 3 or 4 (%) Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: IMFINZI (range: 258 to 263) and chemotherapy (range: 253 to 262) except magnesium IMFINZI with chemotherapy (18) and chemotherapy (16). | Grade 3 or 4 (%) |
Chemistry | ||
Hyponatremia | 11 | 13 |
Hypomagnesemia | 11 | 6 |
Hyperglycemia | 5 | 5 |
Increased Alkaline Phosphatase | 4.9 | 3.5 |
Increased ALT | 4.9 | 2.7 |
Increased AST | 4.6 | 1.2 |
Hypocalcemia | 3.5 | 2.4 |
Blood creatinine increased | 3.4 | 1.1 |
Hyperkalemia | 1.5 | 3.1 |
TSH decreased < LLN LLN = lower limit of normal. and ≥ LLN at baseline | NA | NA |
Hematology | ||
Neutropenia | 41 | 48 |
Lymphopenia | 14 | 13 |
Anemia | 13 | 22 |
Thrombocytopenia | 12 | 15 |
Biliary Tract Cancer
Locally Advanced or Metastatic BTC - TOPAZ-1
The safety of IMFINZI in combination with gemcitabine and cisplatin in locally advanced or metastatic BTC was evaluated in TOPAZ-1, a randomized, double-blind, placebo-controlled, multicenter study. A total of 338 patients received IMFINZI 1,500 mg in combination with gemcitabine and cisplatin every 3 weeks up to 8 cycles followed by IMFINZI 1,500 mg every 4 weeks until disease progression or unacceptable toxicity. Patients with active or prior documented autoimmune or inflammatory disorders, HIV infection or other active infections, including tuberculosis or hepatitis C were ineligible [see Clinical Studies (14.3) ].
IMFINZI was discontinued due to adverse reactions in 6% of the patients receiving IMFINZI plus chemotherapy. The most frequently reported events resulting in discontinuation were sepsis (3 patients) and ischemic stroke (2 patients). The remaining events were dispersed across system organ classes and reported in 1 patient each. Serious adverse reactions occurred in 47% of patients receiving IMFINZI plus chemotherapy. The most frequent serious adverse reactions reported in at least 2% of patients were cholangitis (7%), pyrexia (3.8%), anemia (3.6%), sepsis (3.3%) and acute kidney injury (2.4%). Fatal adverse reactions occurred in 3.6% of patients receiving IMFINZI plus chemotherapy. These include ischemic or hemorrhagic stroke (4 patients), sepsis (2 patients) and upper gastrointestinal hemorrhage (2 patients). The most common adverse reactions (occurring in ≥ 20% of patients) were fatigue, nausea, constipation, decreased appetite, abdominal pain, rash and pyrexia. Table 15 summarizes the adverse reactions that occurred in patients treated with IMFINZI plus chemotherapy.
IMFINZI with Gemcitabine and Cisplatin N = 338 | Placebo with Gemcitabine and Cisplatin N = 342 | |||
Adverse Reaction | All Grades Graded according to NCI CTCAE version 5.0. (%) | Grade 3-4 (%) | All Grades (%) | Grade 3-4 (%) |
General disorders and administration site conditions | ||||
Fatigue Includes fatigue, malaise, cancer fatigue and asthenia. | 42 | 6 | 43 | 6 |
Pyrexia | 20 | 1.5 | 16 | 0.6 |
Gastrointestinal disorders | ||||
Nausea | 40 | 1.5 | 34 | 1.8 |
Constipation | 32 | 0.6 | 29 | 0.3 |
Abdominal pain Includes abdominal pain, abdominal pain lower, abdominal pain upper and flank pain. | 24 | 0.6 | 23 | 2.9 |
Vomiting | 18 | 1.5 | 18 | 2.0 |
Diarrhea | 17 | 1.2 | 15 | 1.8 |
Metabolism and nutrition disorders | ||||
Decreased appetite | 26 | 2.1 | 23 | 0.9 |
Skin and subcutaneous tissue disorders | ||||
Rash Includes rash macular, rash maculopapular, rash morbilliform, rash papular, rash pruritic, rash pustular, rash erythematous, dermatitis acneiform, dermatitis bullous, drug eruption, eczema, erythema, dermatitis and rash. | 23 | 0.9 | 14 | 0 |
Pruritus | 11 | 0 | 8 | 0 |
Psychiatric disorders | ||||
Insomnia | 10 | 0 | 11 | 0 |
Table 16 summarizes the laboratory abnormalities in patients treated with IMFINZI plus chemotherapy.
IMFINZI with Gemcitabine and Cisplatin | Placebo with Gemcitabine and Cisplatin | |
Laboratory Abnormality | Grade Graded according to NCI CTCAE version 5.0. Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: IMFINZI with gemcitabine/cisplatin (range: 312 to 335) and Placebo with gemcitabine/cisplatin (range: 319 to 341). 3 or 4 (%) | Grade 3 or 4 (%) |
Chemistry | ||
Hyponatremia | 18 | 13 |
Gamma-glutamyltransferase increased | 12 | 13 |
Increased bilirubin | 10 | 14 |
Hypokalemia | 8 | 4.4 |
Increased AST | 8 | 8 |
Increased ALT | 7 | 6 |
Blood creatinine increased | 5 | 2.1 |
Hypomagnesemia | 4.5 | 2.2 |
Hypoalbuminemia | 3.6 | 2.9 |
Hyperkalemia | 2.1 | 2.1 |
Increased Alkaline Phosphatase | 1.8 | 3.8 |
Hypocalcemia | 1.8 | 2.4 |
Hematology | ||
Neutropenia | 48 | 49 |
Anemia | 31 | 28 |
Leukopenia | 28 | 28 |
Lymphopenia | 23 | 15 |
Thrombocytopenia | 18 | 18 |
Hepatocellular Carcinoma
Unresectable HCC - HIMALAYA
The safety of IMFINZI in combination with tremelimumab-actl was evaluated in a total of 388 patients with uHCC in HIMALAYA, a randomized, open-label, multicenter study [see Clinical Studies (14.1) ]. Patients received IMFINZI 1,500 mg administered as a single intravenous infusion in combination with tremelimumab-actl 300 mg on the same day, followed by IMFINZI every 4 weeks or sorafenib 400 mg given orally twice daily.
Serious adverse reactions occurred in 41% of patients who received IMFINZI in combination with tremelimumab-actl. Serious adverse reactions in > 1% of patients included hemorrhage (6%), diarrhea (4%), sepsis (2.1%), pneumonia (2.1%), rash (1.5%), vomiting (1.3%), acute kidney injury (1.3%), and anemia (1.3%). Fatal adverse reactions occurred in 8% of patients who received IMFINZI in combination with tremelimumab-actl, including death (1%), hemorrhage intracranial (0.5%), cardiac arrest (0.5%), pneumonitis (0.5%), hepatic failure (0.5%), and immune-mediated hepatitis (0.5%). The most common adverse reactions (occurring in ≥ 20% of patients) were rash, diarrhea, fatigue, pruritus, musculoskeletal pain, and abdominal pain.
Permanent discontinuation of treatment regimen due to an adverse reaction occurred in 14% of patients; the most common adverse reactions leading to treatment discontinuation (≥ 1%) were hemorrhage (1.8%), diarrhea (1.5%), AST increased (1%), and hepatitis (1%).
Dosage interruptions or delay of the treatment regimen due to an adverse reaction occurred in 35% of patients. Adverse reactions which required dosage interruption or delay in ≥ 1% of patients included ALT increased (3.6%), diarrhea (3.6%), rash (3.6%), amylase increased (3.4%), AST increased (3.1%), lipase increased (2.8%), pneumonia (1.5%), hepatitis (1.5%), pyrexia (1.5%), anemia (1.3%), thrombocytopenia (1%), hyperthyroidism (1%), pneumonitis (1%), and blood creatinine increased (1%).
Table 17 summarizes the adverse reactions that occurred in patients treated with IMFINZI in combination with tremelimumab-actl in the HIMALAYA study.
IMFINZI and Tremelimumab-actl (N = 388) | Sorafenib (N = 374) | |||
Adverse Reaction | All Grades (%) | Grade 3-4 (%) | All Grades (%) | Grade 3-4 (%) |
Skin and subcutaneous tissue disorders | ||||
Rash Represents a composite of multiple related terms. | 32 | 2.8 | 57 | 12 |
Pruritus | 23 | 0 | 6 | 0.3 |
Gastrointestinal disorders | ||||
Diarrhea | 27 | 6 | 45 | 4.3 |
Abdominal pain | 20 | 1.8 | 24 | 4 |
Nausea | 12 | 0 | 14 | 0 |
General disorders and administration site conditions | ||||
Fatigue | 26 | 3.9 | 30 | 6 |
Pyrexia | 13 | 0.3 | 9 | 0.3 |
Musculoskeletal and Connective Tissue Disorders | ||||
Musculoskeletal pain | 22 | 2.6 | 17 | 0.8 |
Metabolism and nutrition disorders | ||||
Decreased appetite | 17 | 1.3 | 18 | 0.8 |
Endocrine disorders | ||||
Hypothyroidism | 14 | 0 | 6 | 0 |
Psychiatric disorders | ||||
Insomnia | 10 | 0.3 | 4.3 | 0 |
Table 18 summarizes the laboratory abnormalities that occurred in patients treated with IMFINZI in combination with tremelimumab-actl in the HIMALAYA study.
IMFINZI and Tremelimumab-actl | Sorafenib | |||
Laboratory Abnormality | Any grade Graded according to NCI CTCAE version 4.03. (%) Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: IMFINZI with tremelimumab-actl (range: 367-378) and sorafenib (range:344-352). | Grade 3 or 4 (%) | Any grade (%) | Grade 3 or 4 (%) |
Chemistry | ||||
Aspartate Aminotransferase increased | 63 | 27 | 55 | 21 |
Alanine Aminotransferase increased | 56 | 18 | 53 | 12 |
Sodium decreased | 46 | 15 | 40 | 11 |
Bilirubin increased | 41 | 8 | 47 | 11 |
Alkaline Phosphatase increased | 41 | 8 | 44 | 5 |
Glucose increased | 39 | 14 | 29 | 4 |
Calcium decreased | 34 | 0 | 43 | 0.3 |
Albumin decreased | 31 | 0.5 | 37 | 1.7 |
Potassium increased | 28 | 3.8 | 21 | 2.6 |
Creatinine increased | 21 | 1.3 | 15 | 0.9 |
Hematology | ||||
Hemoglobin decreased | 52 | 4.8 | 40 | 6 |
Lymphocytes decreased | 41 | 11 | 39 | 10 |
Platelets decreased | 29 | 1.6 | 35 | 3.1 |
Leukocytes decreased | 20 | 0.8 | 30 | 1.1 |
Endometrial Cancer
Advanced or Recurrent dMMR Endometrial Cancer – DUO-E
The safety of IMFINZI in combination with carboplatin and paclitaxel followed by IMFINZI as a single agent was evaluated in 44 patients with dMMR advanced or recurrent endometrial cancer in DUO-E, a randomized, double-blind, placebo-controlled trial [See Clinical Studies (14.5) ]. Patients received IMFINZI 1,120 mg with carboplatin and paclitaxel every 3 weeks for up to six 21-day cycles followed by IMFINZI 1,500 mg every 4 weeks or carboplatin and paclitaxel every 3 weeks for up to six 21-day cycles alone. Treatment was continued until disease progression or unacceptable toxicity. The median duration of exposure to IMFINZI with carboplatin and paclitaxel was 14.8 months (range: 0.7 to 31.7).
Serious adverse reactions occurred in 30% of patients who received IMFINZI with carboplatin and paclitaxel. The most common serious adverse reactions (≥4%) were constipation (4.5%) and rash (4.5%).
Permanent discontinuation of IMFINZI due to adverse reactions occurred in 11% of patients. The adverse reaction which resulted in permanent discontinuation of IMFINZI (≥4%) was rash (4.5%).
Dosage interruptions of IMFINZI due to adverse reactions occurred in 52% of patients. Adverse reactions which required dosage interruptions of IMFINZI (≥ 4%) were anemia (11%), thrombocytopenia (9%), neutropenia (9%), COVID-19 (9%), increased ALT (4.5%), and pneumonitis (4.5%).
The most common adverse reactions (> 20%), including laboratory abnormalities, were peripheral neuropathy, musculoskeletal pain, nausea, alopecia, fatigue, abdominal pain, constipation, rash, decreased magnesium, increased ALT, increased AST, diarrhea, vomiting, cough, decreased potassium, dyspnea, headache, increased alkaline phosphatase, and decreased appetite.
Tables 19 and 20 summarize adverse reactions and laboratory abnormalities in DUO-E, respectively.
Adverse Reactions | IMFINZI with Carboplatin and Paclitaxel (N=44) | Carboplatin and Paclitaxel (N=46) | ||
All Grades (%) | Grade 3-4 (%) | All Grades (%) | Grade 3-4 (%) | |
Nervous system disorders | ||||
Peripheral neuropathy Includes neuropathy peripheral, peripheral sensory neuropathy, hypoasthesia, peripheral motor neuropathy, and parasthesia. | 61 | 2.3 | 61 | 4.3 |
Headache | 23 | 0 | 17 | 0 |
Musculoskeletal and connective tissue disorders | ||||
Musculoskeletal pain Includes arthralgia, pain in extremity, back pain, non-cardiac chest pain, myalgia, musculoskeletal pain, musculoskeletal chest pain, arthritis, bone pain, musculoskeletal stiffness, neck pain, musculoskeletal discomfort, and spinal pain. | 59 | 2.3 | 52 | 2.2 |
Gastrointestinal disorders | ||||
Nausea | 59 | 0 | 48 | 2.2 |
Abdominal pain Includes abdominal pain, abdominal pain lower, flank pain, abdominal discomfort, and abdominal pain upper. | 39 | 0 | 24 | 2.2 |
Constipation Includes constipation and fecaloma. | 39 | 4.5 | 35 | 2.2 |
Diarrhea | 27 | 2.3 | 24 | 2.2 |
Vomiting | 27 | 0 | 22 | 4.3 |
Skin and subcutaneous tissue disorders | ||||
Alopecia | 52 | 0 | 41 | 0 |
Rash Includes eczema, rash, rash erythematous, rash maculo-papular, dermatitis, rash pustular, skin exfoliation, and symmetrical drug-related intertriginous, and flexural exanthema. | 39 | 2.3 | 17 | 2.2 |
Pruritus | 16 | 0 | 11 | 0 |
General disorders and administration site conditions | ||||
| 41 | 4.5 | 57 | 11 |
Peripheral edema Includes peripheral edema, peripheral swelling, and edema. | 16 | 0 | 13 | 2.2 |
Respiratory, thoracic and mediastinal disorders | ||||
Cough / productive cough | 27 | 0 | 20 | 0 |
Dyspnea Includes dyspnea and exertional dyspnea. | 25 | 2.3 | 9 | 0 |
Metabolism and nutrition disorders | ||||
Decreased appetite | 18 | 0 | 18 | 0 |
Infections and infestations | ||||
Upper respiratory tract infection Includes nasopharyngitis, pharyngitis, rhinitis, sinusitis, tracheobronchitis, and upper respiratory tract infection. | 14 | 0 | 4.3 | 0 |
Endocrine disorders | ||||
Hypothyroidism Includes blood thyroid stimulating hormone increased, and hypothyroidism. | 11 | 0 | 4.3 | 0 |
Clinically relevant adverse reactions in < 10% of patients who received IMFINZI with carboplatin and paclitaxel included autoimmune hemolytic anemia, colitis, immune-mediated thyroiditis, infusion related reaction, interstitial lung disease, myositis, pneumonitis, pulmonary embolism, and sepsis.
Table 20 summarizes the laboratory abnormalities that occurred in patients treated with IMFINZI with carboplatin and paclitaxel followed by IMFINZI as a single agent.
Laboratory Abnormality | IMFINZI with Carboplatin and Paclitaxel Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: IMFINZI with carboplatin and paclitaxel (range: 40 to 44), and carboplatin and paclitaxel (range: 37 to 46) | Carboplatin and Paclitaxel | |||
All Grades (%) | Grade 3-4 (%) | All Grades (%) | Grade 3-4 (%) | ||
Chemistry | |||||
Magnesium decreased | 36 | 0 | 30 | 2.5 | |
ALT increased | 32 | 2.3 | 22 | 2.2 | |
AST increased | 30 | 2.3 | 22 | 0 | |
Potassium decreased | 25 | 0 | 24 | 2.2 | |
Alkaline phosphatase increased | 20 | 0 | 16 | 0 | |
Muscle Invasive Bladder Cancer (MIBC)
Neoadjuvant and adjuvant treatment of MIBC – NIAGARA
The safety of IMFINZI in combination with neoadjuvant gemcitabine and cisplatin followed by surgery and continued IMFINZI treatment as adjuvant, single-agent therapy was evaluated in NIAGARA, a randomized, open-label, multicenter trial. Patients received IMFINZI in combination with chemotherapy (n=530) or received chemotherapy alone (n=526) [see Clinical Studies (14.6) ] .
The median duration of exposure to IMFINZI 1,500 mg every 3 weeks in the neoadjuvant phase was 12 weeks (range: 1.1 to 84 weeks). The median duration of exposure to IMFINZI 1,500 mg every 4 weeks in the adjuvant phase was 32 weeks (range: 2.4 to 50 weeks).
The most common adverse reactions, including laboratory abnormalities, in the overall study (occurring in ≥ 20% of patients) were decreased hemoglobin, decreased neutrophils, increased blood creatinine, decreased sodium, nausea, increased ALT, decreased calcium, decreased platelets, fatigue, increased potassium, decreased lymphocytes, increased AST, constipation, decreased magnesium, decreased appetite, increased alkaline phosphate, rash, pyrexia, diarrhea, vomiting, and abdominal pain.
Table 21 summarizes the adverse reactions that occurred in patients treated with IMFINZI plus chemotherapy.
IMFINZI with gemcitabine and cisplatin N = 530 | Gemcitabine and cisplatin N = 526 | |||
Adverse Reaction | All Grades Graded according to NCI CTCAE version 5.0. (%) | Grade 3-4 (%) | All Grades (%) | Grade 3-4 (%) |
Gastrointestinal disorders | ||||
Nausea | 54 | 1.5 | 48 | 1 |
Constipation | 39 | 0.8 | 39 | 0.8 |
Diarrhea | 21 | 1.5 | 14 | 0.4 |
Vomiting Includes multiple similar terms. | 20 | 0.9 | 19 | 0.2 |
Abdominal pain | 20 | 0.9 | 13 | 1 |
General disorders and administration site conditions | ||||
Fatigue | 52 | 2.3 | 49 | 3 |
Pyrexia | 22 | 0.4 | 17 | 0 |
Edema | 13 | 0.4 | 13 | 0 |
Metabolism and nutrition disorders | ||||
Decreased appetite | 27 | 0.6 | 25 | 0.6 |
Skin and subcutaneous tissue disorders | ||||
Rash | 23 | 1.3 | 12 | 0.6 |
Pruritus | 15 | 0 | 7 | 0 |
Nervous system disorders | ||||
Peripheral neuropathy | 16 | 0.2 | 14 | 0 |
Headache | 11 | 0 | 11 | 0 |
Dizziness | 11 | 0 | 10 | 0.2 |
Endocrine disorders | ||||
Hypothyroidism | 13 | 0.4 | 2.3 | 0 |
Vascular disorders | ||||
Hypertension | 12 | 4.5 | 9 | 2.9 |
Hemorrhage | 11 | 0.9 | 10 | 2.1 |
Table 22 summarizes the laboratory abnormalities in patients treated with IMFINZI plus chemotherapy.
Laboratory Abnormality | IMFINZI with gemcitabine and cisplatin Each test incidence is based on the number of patients who had both baseline and at least one on-study laboratory measurement available: IMFINZI plus chemotherapy (range: 482 to 528), and chemotherapy (range: 467 to 521). | Gemcitabine and cisplatin | ||
All Grades Graded per NCI CTCAE V5. (%) | Grade 3 or 4 (%) | All Grades (%) | Grade 3 or 4 (%) | |
Chemistry | ||||
Increased blood creatinine | 63 | 9 | 58 | 7 |
Decreased sodium | 54 | 9 | 55 | 10 |
Increased ALT | 53 | 2.3 | 54 | 4.0 |
Decreased calcium | 52 | 1.3 | 43 | 1.2 |
Increased potassium | 51 | 4.2 | 49 | 4.4 |
Increased AST | 42 | 1.5 | 39 | 2.1 |
Decreased magnesium | 38 | 1.9 | 37 | 2.3 |
Increased alkaline phosphate | 26 | 0.8 | 25 | 0.4 |
Hematology | ||||
Decreased hemoglobin | 88 | 13 | 87 | 13 |
Decreased neutrophils | 76 | 31 | 74 | 34 |
Decreased platelets | 52 | 6 | 50 | 7 |
Decreased lymphocytes | 44 | 10 | 40 | 8 |
Neoadjuvant Phase of NIAGARA
A total of 530 patients received at least 1 dose of IMFINZI in combination with chemotherapy as neoadjuvant treatment in the IMFINZI treatment arm, and 526 patients received at least 1 dose of chemotherapy as neoadjuvant treatment in the chemotherapy treatment arm. In the neoadjuvant phase, serious adverse reactions occurred in 24% of patients who received IMFINZI in combination with chemotherapy; the most frequent (≥1%) serious adverse reactions were pulmonary embolism (1.9%), febrile neutropenia (1.5%), acute kidney injury (1.3%), thrombocytopenia (1.3%), urinary tract infection (1.3%) and pneumonia (1.3%). Fatal adverse reactions occurred in 1.1% of patients including sepsis (0.2%), myocardial infarction (0.2%), and pulmonary embolism (0.2%). One fatal adverse reaction of pneumonia was reported in 1 (0.2%) patient in the post-surgery phase before adjuvant treatment started.
Permanent discontinuation of IMFINZI due to an adverse reaction in the neoadjuvant phase occurred in 9% of patients while receiving IMFINZI in combination with chemotherapy. The most frequent (≥ 0.5%) adverse reactions that led to permanent discontinuation of IMFINZI were blood creatinine increased (0.9%), neutropenia (0.6%), acute kidney injury (0.6%), asthenia (0.6%) and fatigue (0.6%).
Of the 530 patients in the IMFINZI treatment arm and 526 patients in the chemotherapy treatment arm who received neoadjuvant treatment, 1 (0.2%) patient in each treatment arm did not receive surgery due to adverse reactions. The adverse reaction that led to cancellation of surgery in the IMFINZI treatment arm was interstitial lung disease.
Of the 469 patients in the IMFINZI treatment arm who underwent radical cystectomy, 4 (0.8%) patients experienced delay of surgery (defined as occurring more than 56 days after the last dose of neoadjuvant treatment) due to adverse reactions.
Adjuvant Phase of NIAGARA
A total of 383 patients (72%) in the IMFINZI treatment arm received at least 1 dose of adjuvant treatment.
Serious adverse reactions occurred in 26% of patients receiving IMFINZI as adjuvant treatment. The most frequent serious adverse reactions (occurring in ≥1% of patients) were urinary tract infection (7%), acute kidney injury (3.7%), hydronephrosis (2.1%), pyelonephritis (2.1%), urosepsis (1.8%), and sepsis (1.6%). Fatal adverse reactions occurred in 1.8% of patients, including COVID-19 (0.3%), severe acute respiratory syndrome (0.3%), cardiopulmonary failure (0.3%), gastrointestinal haemorrhage (0.3%), and chronic hepatic failure (0.3%).
Permanent discontinuation of adjuvant IMFINZI due to an adverse reaction occurred in 5% of patients. The most frequent (≥0.5%) adverse reactions that led to permanent discontinuation of adjuvant IMFINZI were nephritis (0.8%), fatigue (0.5%), diarrhea (0.5%), decreased appetite (0.5%) and pneumonitis (0.5%).
Gastric or Gastroesophageal Junction Adenocarcinoma (GC/GEJC)
Neoadjuvant and Adjuvant Treatment of Resectable GC/GEJC – MATTERHORN
The safety of IMFINZI with FLOT as neoadjuvant and adjuvant treatment, followed by single-agent IMFINZI, was evaluated in MATTERHORN, a randomized, double-blind, placebo-controlled, multicenter study of patients with resectable GC/GEJC (Stage II to Stage IVA [AJCC, 8th edition]) [see Clinical Studies (14.7 )] .
Safety data are available for the 944 patients who received IMFINZI with FLOT (n=475) or placebo with FLOT (n=469).
The median duration of exposure to IMFINZI 1,500 mg every 4 weeks in the neoadjuvant phase was 8 weeks (range: 1.4 to 8.7 weeks). The median duration of exposure to IMFINZI 1,500 mg every 4 weeks in the adjuvant phase was 48 weeks (range: 1.9 to 50.1 weeks).
The most common adverse reactions (occurring in ≥ 20% of patients) were diarrhea, nausea, peripheral neuropathy, fatigue, alopecia, decreased appetite, rash, abdominal pain, vomiting, musculoskeletal pain, pyrexia, and stomatitis.
Table 23 summarizes the adverse reactions that occurred in ≥ 10% of patients treated with IMFINZI in combination with FLOT chemotherapy.
Adverse Reaction | IMFINZI with FLOT Chemotherapy N=475 | Placebo with FLOT Chemotherapy N=469 | ||
All Grades (%) | Grade 3 or 4 (%) | All Grades (%) | Grade 3 or 4 (%) | |
Gastrointestinal disorders | ||||
Diarrhea Includes multiple similar terms. | 64 | 8 | 59 | 7 |
Nausea | 51 | 2.5 | 51 | 1.5 |
Vomiting | 26 | 2.1 | 26 | 3 |
Abdominal pain | 27 | 2.3 | 29 | 1.3 |
Stomatitis | 20 | 1.1 | 15 | 0.6 |
Constipation | 16 | 0 | 17 | 0.9 |
Dysphagia | 10 | 1.5 | 8 | 1.5 |
Nervous system disorders | ||||
Peripheral neuropathy | 51 | 3.6 | 47 | 2.3 |
Dysgeusia | 19 | 0 | 15 | 0 |
General disorders and administration site conditions | ||||
Fatigue | 47 | 5 | 45 | 5 |
Pyrexia | 20 | 0.8 | 16 | 1.3 |
Skin and subcutaneous tissue disorders | ||||
Alopecia | 31 | 0 | 32 | 0 |
Rash | 30 | 1.5 | 20 | 0.4 |
Pruritus | 11 | 0 | 5 | 0 |
Metabolism and nutrition disorders | ||||
Decreased appetite | 31 | 3.2 | 30 | 2.1 |
Musculoskeletal and connective tissue disorders | ||||
Musculoskeletal pain | 21 | 1.5 | 19 | 0.6 |
Infections and infestations | ||||
COVID-19 | 19 | 1.7 | 16 | 0.6 |
Pneumonia | 11 | 3.8 | 10 | 4.3 |
Investigations | ||||
Weight decreased | 15 | 2.1 | 19 | 3.4 |
Vascular disorders | ||||
Hemorrhage | 14 | 2.5 | 15 | 2.8 |
Respiratory, thoracic and mediastinal disorders | ||||
Cough | 10 | 0 | 10 | 0 |
- Table 24 summarizes the laboratory abnormalities in patients treated with IMFINZI in combination with FLOT chemotherapy.
Laboratory Abnormality Graded per NCI CTCAE v5.0 | IMFINZI with FLOT Chemotherapy The denominator used to calculate the rate varied from 444 to 472 based on the number of patients with a baseline value and at least one post-treatment value. | Placebo with FLOT Chemotherapy The denominator used to calculate the rate varied from 443 to 468 based on the number of patients with a baseline value and at least one post-treatment value. | ||
All Grades (%) | Grade 3 or 4 (%) | All Grades (%) | Grade 3 or 4 (%) | |
Hematology | ||||
Decreased leukocytes | 75 | 14 | 79 | 17 |
Decreased neutrophils | 70 | 41 | 74 | 44 |
Decreased hemoglobin | 68 | 7 | 73 | 8 |
Decreased lymphocytes | 49 | 13 | 45 | 13 |
Decreased platelets | 47 | 1.1 | 46 | 1.9 |
Chemistry | ||||
Increased AST | 72 | 6 | 69 | 6 |
Increased ALT | 70 | 7 | 65 | 6 |
Increased GGT | 57 | 10 | 51 | 6 |
Increased lipase | 55 | 16 | 55 | 15 |
Increased alkaline phosphatase | 53 | 3.2 | 51 | 1.5 |
Decreased calcium | 45 | 0.8 | 47 | 2.6 |
Increased amylase | 42 | 6 | 39 | 4.5 |
Decreased potassium | 39 | 8 | 40 | 8 |
Decreased sodium | 33 | 4.4 | 32 | 4.7 |
Decreased albumin | 31 | 1.5 | 31 | 0.2 |
Increased potassium | 22 | 0.6 | 26 | 1.3 |
Adverse Reactions by Phase of Treatment
Table 25 summarizes safety profile by Phase of treatment.
IMFINZI with FLOT Chemotherapy | Placebo with FLOT Chemotherapy | |
Neoadjuvant Phase | ||
Number of Patients | 475 | 469 |
Serious Adverse Reactions | 21% | 18% |
Deaths during Treatment-emergent period | 1.9% | 1.7% |
| 2.5% | 2.1% |
No surgery due to Adverse Reaction | 0.6% | 0.4% |
Delay in surgery due to Adverse Reaction | 2.3% | 2.6% |
Adjuvant Phase | ||
Number of Patients | 365 | 351 |
Serious Adverse Reactions | 29% | 26% |
Deaths during Treatment-emergent period | 2.2% | 2.6% |
| 7% | 4.6% |
Adjuvant Phase (IMFINZI as a single-agent) | ||
Number of Patients | 345 | 331 |
Serious Adverse Reactions | 14% | 15% |
Deaths during Treatment-emergent period | 1.7% | 2.4% |
| 6% | 2.7% |
Serious adverse reactions (≥2%) in the IMFINZI arm were diarrhea (2.5%) during the neoadjuvant phase, and pneumonia (2.5%) in the adjuvant phase.
Deaths (≥2 patients) in the IMFINZI arm were septic shock (0.6%) and acute coronary syndrome (0.4%) during the neoadjuvant phase; gastrointestinal perforation (0.5%) and COVID-19 (0.5%) during adjuvant phase; and gastrointestinal perforation (0.6%) and COVID-19 (0.6%) in the single-agent adjuvant phase.
Permanent discontinuation of IMFINZI (≥2 patients) due an adverse reaction in the IMFINZI arm were nephritis (0.4%) during the neoadjuvant phase; hepatitis (1.1%), pneumonitis (1.1%), rash (0.8%), musculoskeletal pain (0.5%), and renal failure (0.5%) during adjuvant phase; and hepatitis (0.9%), pneumonitis (0.9%), musculoskeletal pain (0.6%), and renal failure (0.6%) during the single-agent adjuvant phase.
DESCRIPTION
Durvalumab is a programmed cell death ligand 1 (PD-L1) blocking antibody. Durvalumab is a human immunoglobulin G1 kappa (IgG1κ) monoclonal antibody that is produced by recombinant DNA technology in Chinese Hamster Ovary (CHO) cell suspension culture.
IMFINZI (durvalumab) Injection for intravenous use is a sterile, preservative-free, clear to opalescent, colorless to slightly yellow solution, free from visible particles.
Each 500 mg vial of IMFINZI contains 500 mg of durvalumab in 10 mL solution. Each mL contains durvalumab, 50 mg, L-histidine (2 mg), L-histidine hydrochloride monohydrate (2.7 mg), α,α-trehalose dihydrate (104 mg), Polysorbate 80 (0.2 mg), and Water for Injection, USP.
Each 120 mg vial of IMFINZI contains 120 mg of durvalumab in 2.4 mL solution. Each mL contains durvalumab, 50 mg, L-histidine (2 mg), L-histidine hydrochloride monohydrate (2.7 mg), α,α-trehalose dihydrate (104 mg), Polysorbate 80 (0.2 mg), and Water for Injection, USP.
CLINICAL PHARMACOLOGY
Mechanism of Action
Expression of programmed cell death ligand-1 (PD-L1) can be induced by inflammatory signals (e.g., IFN-gamma) and can be expressed on both tumor cells and tumor-associated immune cells in the tumor microenvironment. PD-L1 blocks T-cell function and activation through interaction with PD-1 and CD80 (B7.1). By binding to its receptors, PD-L1 reduces cytotoxic T-cell activity, proliferation, and cytokine production.
Durvalumab is a human immunoglobulin G1 kappa (IgG1κ) monoclonal antibody that binds to PD-L1 and blocks the interaction of PD-L1 with PD-1 and CD80 (B7.1). Blockade of PD-L1/PD-1 and PD-L1/CD80 interactions releases the inhibition of immune responses, without inducing antibody dependent cell-mediated cytotoxicity (ADCC).
PD-L1 blockade with durvalumab led to increased T-cell activation in vitro and decreased tumor size in co-engrafted human tumor and immune cell xenograft mouse models.
Pharmacodynamics
The steady state AUC, Ctrough, and C max in patients administered with 1,500 mg every 4 weeks are 6% higher, 19% lower, and 55% higher than those administered with 10 mg/kg every 2 weeks, respectively. Based on the modeling of pharmacokinetic data and exposure relationships for safety, there are no anticipated clinically meaningful differences in efficacy and safety for the doses of 1,500 mg every 4 weeks compared to 10 mg/kg every 2 weeks in patients weighing > 30 kg with NSCLC.
Pharmacokinetics
The pharmacokinetics of durvalumab as a single agent was studied in patients with doses ranging from 0.1 mg/kg (0.01 times the approved recommended dosage) to 20 mg/kg (2 times the approved recommended dosage) administered once every two, three, or four weeks.
PK exposure increased more than dose-proportionally at doses < 3 mg/kg (0.3 times the approved recommended dosage) and dose proportionally at doses ≥ 3 mg/kg every 2 weeks. Steady state was achieved at approximately 16 weeks.
The pharmacokinetics of durvalumab is similar when assessed as a single agent, when in combination with chemotherapy, when in combination with tremelimumab-actl and when in combination with tremelimumab-actl and platinum-based chemotherapy.
Distribution
The geometric mean (% coefficient of variation [CV%]) steady state volume of distribution (Vss) was 5.4 (13.1%) L.
Elimination
Durvalumab clearance decreases over time, with a mean maximal reduction (CV%) from baseline values of approximately 23% (57%) resulting in a geometric mean (CV%) steady state clearance (CLss) of 8 mL/h (39%) at day 365; the decrease in CLss is not considered clinically relevant. The geometric mean (CV%) terminal half-life, based on baseline CL was approximately 21 (26%) days.
Specific Populations
There were no clinically significant differences in the pharmacokinetics of durvalumab based on body weight (31 to 175 kg), age (18 to 96 years), sex, race (White, Black, Asian, Native Hawaiian, Pacific Islander, or Native American), albumin levels (4 to 57 g/L), lactate dehydrogenase levels (18 to 15,800 U/L), soluble PD-L1 (67 to 3,470 pg/mL), tumor type (NSCLC, SCLC, BTC and HCC), mild or moderate renal impairment (CLcr 30 to 89 mL/min), and mild or moderate hepatic impairment (bilirubin ≤ 3x ULN and any AST). The effect of severe renal impairment (CLcr 15 to 29 mL/min) or severe hepatic impairment (bilirubin > 3x ULN and any AST) on the pharmacokinetics of durvalumab is unknown.
Immunogenicity
The observed incidence of anti-drug antibodies (ADA) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparison of the incidence of ADAs in the studies described below with the incidence of ADAs in other studies including those of IMFINZI or of other durvalumab products.
In clinical trials of patients who received IMFINZI for 10 to 48 weeks at dosages of 1,500 mg every 4 weeks, 10 mg/kg every 2 weeks, 20 mg/kg every 4 weeks as a single agent or 1,120 mg every 3 weeks, or 1,500 mg every 3 weeks in the combination therapies, 3.6% (199/5562) of evaluable patients tested positive for treatment-emergent anti-durvalumab antibodies, and 8.5% (17/199) of treatment-emergent ADA positive patients had neutralizing antibodies against durvalumab. There were no identified clinically significant effects of ADAs on durvalumab pharmacokinetics or safety; however, the effect of these ADAs on the effectiveness of IMFINZI is unknown.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic and genotoxic potential of durvalumab have not been evaluated.
Animal fertility studies have not been conducted with durvalumab. In repeat-dose toxicology studies with durvalumab in sexually mature cynomolgus monkeys of up to 3 months duration, there were no notable effects on the male and female reproductive organs.
Animal Toxicology and/or Pharmacology
In animal models, inhibition of PD-L1/PD-1 signaling increased the severity of some infections and enhanced inflammatory responses. Mycobacterium tuberculosis -infected PD-1 knockout mice exhibit markedly decreased survival compared with wild-type controls, which correlated with increased bacterial proliferation and inflammatory responses in these animals. PD-1 blockade using a primate anti-PD-1 antibody was also shown to exacerbate M. tuberculosis infection in rhesus macaques. PD-L1 and PD-1 knockout mice and mice receiving PD-L1 blocking antibody have also shown decreased survival following infection with lymphocytic choriomeningitis virus.
CLINICAL STUDIES
Non-Small Cell Lung Cancer (NSCLC)
Neoadjuvant and Adjuvant Treatment of Resectable NSCLC – AEGEAN Study
The efficacy of IMFINZI in combination with neoadjuvant chemotherapy, followed by surgery and continued adjuvant treatment with IMFINZI as a single agent was investigated in AEGEAN (NCT03800134), a randomized, double-blind, placebo-controlled, multicenter trial conducted in 802 patients with previously untreated and resectable squamous or non-squamous NSCLC (Stage IIA to select Stage IIIB [AJCC, 8th edition]). Patients were enrolled regardless of tumor PD-L1 expression. Eligible patients had no prior exposure to immune-mediated therapy, a WHO/ECOG Performance status of 0 or 1, and at least one RECIST 1.1 target lesion.
Patients with active or prior documented autoimmune disease, or use of any immunosuppressive medication within 14 days of the first dose of IMFINZI were ineligible. The population for efficacy analyses was a modified intent-to-treat [mITT] which excluded patients with known EGFR mutations or ALK rearrangements.
Crossover between the study arms was not permitted. Randomization was stratified by disease stage (Stage II vs. Stage III) and by PD-L1 expression (TC < 1% vs. TC ≥ 1%) status. Patients were randomized 1:1 to one of the following treatment arms:
- Arm 1: Neoadjuvant IMFINZI 1,500 mg once every 3 weeks for up to 4 cycles in combination with:
Squamous tumor histology: carboplatin AUC 6 and paclitaxel 200 mg/m 2 on Day1 of each 3-week cycle, OR cisplatin 75 mg/m 2 on Day 1 and gemcitabine 1250 mg/m 2 on Day 1 and Day 8 of each 3-week cycle, for 4 cycles
Non-squamous tumor histology: pemetrexed 500 mg/m 2 and cisplatin 75 mg/m 2 on Day 1 of each 3-week cycle, for 4 cycles OR pemetrexed 500 mg/m 2 and carboplatin AUC 5 on Day 1 of each 3-week cycle, for 4 cycles.
Followed by adjuvant IMFINZI 1,500 mg as a single agent for up to 12 cycles post-surgery.
- Arm 2: Neoadjuvant placebo in combination with 4 cycles of chemotherapy (see above) prior to surgery.
- Followed by placebo for up to 12 cycles post-surgery.
All study medications were administered via intravenous infusion. In the event of unfavorable tolerability, patients who met the eligibility criteria were switched from cisplatin to carboplatin therapy at any point during the study. In patients with comorbidities or unable to tolerate cisplatin as per Investigators judgment, carboplatin AUC 5 could be administered from cycle 1. Treatment with IMFINZI or placebo continued until completion of the treatment, disease progression that precluded definitive surgery, inability to complete definitive surgery, disease recurrence in the adjuvant phase, or unacceptable toxicity. A RECIST 1.1 tumor assessment was performed at baseline, and upon completion of the neoadjuvant period (prior to surgery). Tumor assessments were conducted at 5 weeks postoperatively, prior to the start of adjuvant therapy and every 12 weeks until week 48, every 24 weeks for approximately 4 years, and then every 48 weeks thereafter until disease progression, consent withdrawal, or death.
The trial was not designed to isolate the effect of IMFINZI in each phase (neoadjuvant or adjuvant) of treatment.
The major efficacy outcome measures of the study were pathological complete response (pCR) by blinded central pathology review and event-free survival (EFS) by blinded independent central review (BICR) assessment. Additional efficacy outcome measures were major pathological response (MPR) by blinded central pathology review, DFS by BICR, and OS.
The demographics and baseline disease characteristics were as follows: male (72%); median age 65 years (range: 30 to 88); age ≥ 65 years (52%); WHO/ECOG PS 0 (68%), WHO/ECOG PS 1 (32); White (54%), Asian (41%), Black or African American (0.9%), American Indian or Alaska Native (1.4%), Other Race (2.6%); Not Hispanic or Latino (84%); current or past smokers (86%); squamous histology (49%) and non-squamous histology (51%); Stage II (28%), Stage III (71%); PD-L1 expression status TC ≥ 1% (67%), PD-L1 expression status TC < 1% (33%).
In the mITT population, 78% of patients in Arm 1 completed definitive surgery compared to 77% of patients in Arm 2.
The trial demonstrated statistically significant improvements in EFS and pCR rate (see Table 26 and Figure 1) in the IMFINZI in combination with chemotherapy arm compared to the placebo in combination with chemotherapy arm.
| IMFINZI 1,500 mg every 3 weeks with chemotherapy/ IMFINZI (N = 366) | Placebo with Chemotherapy/ Placebo (N = 374) | |
|---|---|---|
EFS Results are based on planned EFS interim analysis and pCR final analysis (DCO: 10 November 2022) which occurred 46.3 months after study initiation. | ||
| 98 (27) | 138 (37) |
| NR (31.9, NR) | 25.9 (18.9, NR) |
| 0.68 (0.53, 0.88) | |
| 0.0039 | |
pCR , Based on a pre-specified pCR interim analysis (DCO: 14 January 2022) in n = 402, the pCR rate was statistically significant (p = 0.000036) compared to significance level of 0.0082%. , | ||
| 63 | 16 |
| 17.2 (13.5, 21.5) | 4.3 (2.5, 6.8) |
| < 0.0001 | |
| 13.0 (8.7, 17.6) | |
Figure 1. Kaplan-Meier Curves of EFS in the AEGEAN Study

At the interim analysis, the trial demonstrated a statistically significant difference in MPR rate (34% vs. 14%; p < 0.0001). At the time of the prespecified interim analyses, overall survival (OS) was not formally tested for statistical significance.
Unresectable Stage III NSCLC - PACIFIC
The efficacy of IMFINZI was evaluated in the PACIFIC study (NCT02125461), a multicenter, randomized, double-blind, placebo-controlled study in patients with unresectable Stage III NSCLC who completed at least 2 cycles of concurrent platinum-based chemotherapy and definitive radiation within 42 days prior to initiation of the study drug and had a WHO performance status of 0 or 1. The study excluded patients who had progressed following concurrent chemoradiation, patients with active or prior documented autoimmune disease within 2 years of initiation of the study or patients with medical conditions that required systemic immunosuppression. Randomization was stratified by sex, age (< 65 years vs. ≥ 65 years), and smoking history (smoker vs. non-smoker). Patients were randomized 2:1 to receive IMFINZI 10 mg/kg or placebo intravenously every 2 weeks for up to 12 months or until unacceptable toxicity or confirmed RECIST v1.1-defined progression. Assessment of tumor status was performed every 8 weeks. The major efficacy outcome measures were progression-free survival (PFS) as assessed by a BICR RECIST v1.1, and overall survival (OS). Additional efficacy outcome measures included ORR and DoR assessed by BICR.
A total of 713 patients were randomized: 476 patients to the IMFINZI arm and 237 to the placebo arm. The study population characteristics were: median age of 64 years (range: 23 to 90); 70% male; 69% White and 27% Asian; 16% current smokers, 75% former smokers, and 9% never smokers; 51% WHO performance status of 1; 53% with Stage IIIA and 45% were Stage IIIB; 46% with squamous and 54% with non-squamous histology. All patients received definitive radiotherapy as per protocol, of which 92% received a total radiation dose of 54 Gy to 66 Gy; 99% of patients received concomitant platinum-based chemotherapy (55% cisplatin-based, 42% carboplatin-based chemotherapy, and 2% switched between cisplatin and carboplatin).
At a pre-specified interim analysis for OS based on 299 events (61% of total planned events), the study demonstrated a statistically significant improvement in OS in patients randomized to IMFINZI compared to placebo. The pre-specified interim analysis of PFS based on 371 events (81% of total planned events) demonstrated a statistically significant improvement in PFS in patients randomized to IMFINZI compared to placebo. Table 27 and Figure 2 summarizes the efficacy results for PACIFIC.
| Endpoint | IMFINZI (N = 476) Among the ITT population, 7% in the IMFINZI arm and 10% in the placebo arm had non-measurable disease as assessed by BICR according to RECIST v1.1 | Placebo (N = 237) |
|---|---|---|
| Overall Survival (OS) OS results are based on the interim OS analysis conducted at 299 OS events which occurred 46 months after study initiation. | ||
| Number of deaths | 183 (38%) | 116 (49%) |
| Median in months (95% CI) | NR (34.7, NR) | 28.7 (22.9, NR) |
| Hazard Ratio (95% CI) Two-sided p-value based on a log-rank test stratified by sex, age, and smoking history | 0.68 (0.53, 0.87) | |
| p-value Compared with allocated α of 0.00274 (Lan‑DeMets spending function approximating O’Brien Fleming boundary) for interim analysis | 0.0025 | |
| ||
|
|
|
|
|
|
|
| |
|
| |
Figure 2. Kaplan-Meier Curves of OS in the PACIFIC Study

Metastatic NSCLC - POSEIDON
The efficacy of IMFINZI in combination with tremelimumab-actl and platinum-based chemotherapy in previously untreated metastatic NSCLC patients with no sensitizing epidermal growth factor receptor (EGFR) mutation or anaplastic lymphoma kinase (ALK) genomic tumor aberrations was investigated in POSEIDON, a randomized, multicenter, active-controlled, open-label study (NCT03164616). Eligible patients had Eastern Cooperative Oncology Group (ECOG) Performance Status of 0 or 1 and must have had no prior chemotherapy or any other systemic therapy for metastatic NSCLC. Choice of platinum-based chemotherapy was at the investigator’s discretion, taking into consideration the calculated creatinine clearance. Patients with active and/or untreated brain metastases; a history of active primary immunodeficiency; autoimmune disorders including active or prior documented autoimmune or inflammatory disorders; use of systemic immunosuppressants within 14 days before the first dose of the treatment except physiological dose of systemic corticosteroids were ineligible.
Randomization was stratified by tumor cells (TC) PD-L1 expression (TC ≥ 50% vs. TC < 50%), disease stage (Stage IVA vs. Stage IVB), and histology (non-squamous vs. squamous).
Patients were randomized 1:1:1 to receive IMFINZI in combination with tremelimumab-actl and platinum-based chemotherapy according to the regimens listed below, IMFINZI and platinum-based chemotherapy (an unapproved regimen for metastatic NSCLC), or platinum-based chemotherapy. The evaluation of efficacy for metastatic NSCLC relied on comparison between:
- IMFINZI 1,500 mg with tremelimumab-actl 75 mg (or 1 mg/kg for patients < 30 kg) and platinum-based chemotherapy every 3 weeks for 4 cycles, followed by IMFINZI 1,500 mg every 4 weeks as a single agent. A fifth dose of tremelimumab-actl 75 mg (or 1 mg/kg for patients < 30 kg) was given at Week 16 in combination with IMFINZI dose 6.
- Platinum-based chemotherapy every 3 weeks as monotherapy for 4 cycles. Patients could receive an additional 2 cycles (a total of 6 cycles post-randomization), as clinically indicated, at investigator’s discretion.
Patients received IMFINZI in combination with tremelimumab-actl with one of the following platinum-based chemotherapy regimens:
- Non-squamous NSCLC
- Pemetrexed 500 mg/m 2 with carboplatin AUC 5-6 or cisplatin 75 mg/m 2 every 3 weeks for 4 cycles.
- Squamous NSCLC
- Gemcitabine 1,000 or 1,250 mg/m 2 on Days 1 and 8 with cisplatin 75 mg/m 2 or carboplatin AUC 5-6 on Day 1 every 3 weeks for 4 cycles.
- Non-squamous and Squamous NSCLC
- Nab-paclitaxel 100 mg/m 2 on Days 1, 8, and 15 with carboplatin AUC 5-6 on Day 1 every 3 weeks for 4 cycles.
Tremelimumab-actl was given up to a maximum of 5 doses. IMFINZI and histology-based pemetrexed continued every 4 weeks until disease progression or unacceptable toxicity. Administration of IMFINZI monotherapy was permitted beyond disease progression if the patient was clinically stable and deriving clinical benefit as determined by the investigator. Patients with disease progression during IMFINZI monotherapy were given the option to be retreated with 4 additional cycles of tremelimumab-actl in combination with IMFINZI. Tumor assessments were performed at Week 6, Week 12, and then every 8 weeks thereafter.
The major efficacy outcome measures were progression free survival (PFS) and overall survival (OS) of IMFINZI and tremelimumab-actl in combination with platinum-based chemotherapy compared to platinum-based chemotherapy alone. Additional efficacy outcome measures were overall response rate (ORR) and duration of response (DoR). PFS, ORR, and DoR were assessed using Blinded Independent Central Review (BICR) according to RECIST v1.1.
A total of 675 patients were randomized to receive either IMFINZI with tremelimumab-actl and platinum-based-chemotherapy (n = 338) or platinum-based chemotherapy (n = 337). The median age was 63 years (range: 27 to 87), 46% of patients age ≥ 65 years, 77% male, 57% White, 34% Asian, 0.3% Native Hawaiian or Other Pacific Islander, 3% American Indian or Alaska Native, 2% Black or African American, 4% Other Race, 79% former or current smoker, 34% ECOG PS 0, and 66% ECOG PS 1. Thirty-six percent had squamous histology, 63% non-squamous histology, 29% PD-L1 expression TC ≥ 50%, 71% PD-L1 expression TC < 50%.
Efficacy results are summarized in Table 28 and Figure 3.
IMFINZI with tremelimumab-actl and platinum-based chemotherapy (N = 338) | Platinum-based chemotherapy (N = 337) | |
OS PFS/OS results are based on planned analyses which occurred 25/45 months respectively after study initiation. | ||
| 251 (74) | 285 (85) |
| 14.0 (11.7, 16.1) | 11.7 (10.5, 13.1) |
| 0.77 (0.65, 0.92) | |
| 0.00304 | |
PFS | ||
| 238 (70) | 258 (77) |
| 6.2 (5.0, 6.5) | 4.8 (4.6, 5.8) |
| 0.72 (0.60, 0.86) | |
| 0.00031 | |
ORR % (95% CI) Confirmed responses with 95% Clopper-Pearson confidence interval. | 39 (34, 44) | 24 (20, 29) |
Median DoR (months)
| 9.5 (7.2, NR) | 5.1 (4.4, 6.0) |
NR=Not Reached, CI=Confidence Interval
Figure 3. Kaplan-Meier Curves of OS in the POSEIDON Study

Small Cell Lung Cancer (SCLC)
Limited-stage SCLC - ADRIATIC
The efficacy of IMFINZI was evaluated in the ADRIATIC Study (NCT03703297), a randomized, double-blind, placebo-controlled, multicenter study in 730 patients with histologically or cytologically confirmed LS-SCLC (Stage I to III according to AJCC, 8th edition) whose disease had not progressed following concurrent chemoradiation therapy (cCRT). Patients who had Stage I or II disease had to be medically inoperable as determined by the investigator. Eligible patients completed cCRT consisting of 4 cycles of platinum-based chemotherapy and either 60-66 Gy once daily over 6 weeks or 45 Gy twice daily over 3 weeks radiation therapy within 42 days prior to the first dose of IMFINZI or placebo. Prophylactic cranial irradiation (PCI) could be delivered at the discretion of the investigator after cCRT and had to be completed within 42 days prior to the first dose of IMFINZI or placebo. Patients with active or prior documented autoimmune disease within 5 years of initiation into the study; a history of active primary immunodeficiency; a history of Grade ≥ 2 pneumonitis or active tuberculosis or hepatitis B or C or HIV infection; active interstitial lung disease were ineligible. Patients with mixed SCLC and NSCLC histology were also ineligible. Randomization was stratified by stage (I/II versus III) and receipt of PCI (yes versus no).
Patients were randomized 1:1:1 to receive IMFINZI as a single agent, IMFINZI in combination with another agent, or placebo. All study medications were administered intravenously. The evaluation of efficacy for LS-SCLC relied on comparison between:
- Arm 1: IMFINZI 1,500 mg in combination with placebo every 4 weeks for 4 cycles, followed by IMFINZI 1,500 mg every 4 weeks.
- Arm 2: Placebo in combination with a second placebo every 4 weeks for 4 cycles, followed by a single placebo every 4 weeks.
A total of 530 patients were randomized between Arms 1 and 2, 264 patients to the IMFINZI arm and 266 patients to the placebo arm. Treatment continued until disease progression, until unacceptable toxicity, or for a maximum of 24 months. Tumor assessments were conducted every 8 weeks for the first 72 weeks, then every 12 weeks up to 96 weeks and then every 24 weeks thereafter.
The major efficacy outcome measures were OS and PFS assessed by BICR according to RECIST v1.1.
The baseline demographics and disease characteristics for patients in the IMFINZI and placebo arms were as follows: male (69%); age ≥ 65 years (39%); White (50%), Black or African-American (0.8%), Asian (48%), other race (1.3%); Hispanic or Latino (4.2%); current smoker (22%), past-smoker (68%), never smoker (9%); WHO/ECOG PS 0 (49%), WHO/ECOG PS 1 (51%); and Stage I (3.6%), Stage II (9%), Stage III (87%).
Prior to randomization, all patients received platinum-based chemotherapy (66% cisplatin-etoposide, 34% carboplatin-etoposide) with 88% of patients receiving 4 cycles; 72% of patients received once daily radiation (of which 92% received ≥ 60 - ≤ 66 Gy QD); 28% received twice daily radiation (of which 97% received 45 Gy twice daily) and 54% of patients received PCI.
Efficacy results are presented in Table 29 and Figure 4.
| IMFINZI (N=264) | Placebo (N=266) | |
|---|---|---|
OS | ||
Number of deaths (%) | 115 (44) | 146 (55) |
Median OS (months) (95% CI) Calculated using the Kaplan Meier technique. CI for median derived based on Brookmeyer-Crowley method. | 55.9 (37.3, NR) | 33.4 (25.5, 39.9) |
HR (95% CI) Based on Cox proportional hazards model stratified by receipt of PCI. | 0.73 (0.57, 0.93) | |
p-value Compared with allocated alpha of 0.0168 for OS and 0.0280 for PFS (Lan‑DeMets spending function approximating O’Brien Fleming boundary) for interim analysis. | 0.0104 | |
PFS Assessed by BICR according to RECIST v1.1. | ||
Number of events (%) | 139 (53) | 169 (64) |
Median PFS (months) (95% CI) | 16.6 (10.2, 28.2) | 9.2 (7.4, 12.9) |
HR (95% CI) Based on Cox proportional hazards model stratified by TNM stage and receipt of PCI. | 0.76 (0.61, 0.95) | |
p-value | 0.0161 | |
Figure 4. Kaplan-Meier Curves of OS in the ADRIATIC Study

Extensive-stage SCLC – CASPIAN
The efficacy of IMFINZI in combination with etoposide and either carboplatin or cisplatin in previously untreated ES-SCLC was investigated in CASPIAN, a randomized, multicenter, active-controlled, open-label study (NCT03043872). Eligible patients had WHO Performance Status of 0 or 1 and were suitable to receive a platinum-based chemotherapy regimen as first-line treatment for SCLC. Patients with asymptomatic or treated brain metastases were eligible. Choice of platinum agent was at the investigator’s discretion, taking into consideration the calculated creatinine clearance. Patients with history of chest radiation therapy; a history of active primary immunodeficiency; autoimmune disorders including paraneoplastic syndrome; active or prior documented autoimmune or inflammatory disorders; use of systemic immunosuppressants within 14 days before the first dose of the treatment except physiological dose of systemic corticosteroids were ineligible.
Randomization was stratified by the planned platinum-based therapy in cycle 1 (carboplatin or cisplatin).
The evaluation of efficacy for ES-SCLC relied on comparison between:
IMFINZI 1,500 mg, and investigator’s choice of carboplatin (AUC 5 or 6 mg/mL/min) or cisplatin (75-80 mg/m 2 ) on Day 1 and etoposide (80-100 mg/m 2 ) intravenously on Days 1, 2, and 3 of each 21-day cycle for 4 cycles, followed by IMFINZI 1,500 mg every 4 weeks until disease progression or unacceptable toxicity, or
Investigator’s choice of carboplatin (AUC 5 or 6 mg/mL/min) or cisplatin (75-80 mg/m 2 ) on Day 1 and etoposide (80-100 mg/m 2 ) intravenously on Days 1, 2, and 3 of each 21-day cycle, up to 6 cycles. After completion of chemotherapy, PCI as administered per investigator discretion.
Administration of IMFINZI as a single agent was permitted beyond disease progression if the patient was clinically stable and deriving clinical benefit as determined by the investigator.
The major efficacy outcome measure was overall survival (OS) of IMFINZI plus chemotherapy vs. chemotherapy alone. Additional efficacy outcome measures were investigator-assessed progression-free survival (PFS) and objective response rate (ORR), per RECIST v1.1.
The study population characteristics were: median age of 63 years (range: 28 to 82); 40% age 65 or older; 70% male; 84% White, 15% Asian, and 0.9% Black; 65% WHO/ECOG PS of 1; and 93% were former/current smokers. Ninety percent of patients had Stage IV disease and 10% had brain metastasis at baseline. A total of 25% of the patients received cisplatin and 74% of the patients received carboplatin. In the chemotherapy alone arm, 57% of the patients received 6 cycles of chemotherapy, and 8% of the patients received PCI.
The OS results are summarized in Table 30 and Figure 5.
Endpoint | IMFINZI with Etoposide and either Carboplatin or Cisplatin (N = 268) | Etoposide and either Carboplatin or Cisplatin (N = 269) |
Overall Survival (OS) | ||
Number of deaths (%) At a pre-specified interim analysis, 336 OS events (79% of total planned events) were observed, and the boundary for declaring efficacy (0.0178) was determined by a Lan-Demets alpha spending function with O’Brien Fleming type boundary. | 155 (58) | 181 (67) |
Median OS (months) (95% CI) | 13.0 (11.5, 14.8) | 10.3 (9.3, 11.2) |
Hazard Ratio (95% CI) The analysis was performed using the stratified log-rank test, adjusting for planned platinum therapy in Cycle 1 (carboplatin or cisplatin) and using the rank tests of association approach. | 0.73 (0.59, 0.91) | |
p-value | 0.0047 | |
Figure 5. Kaplan-Meier Curves of OS in the CASPIAN Study

Investigator-assessed PFS (96% of total planned events) showed a HR of 0.78 (95% CI: 0.65, 0.94), with median PFS of 5.1 months (95% CI: 4.7, 6.2) in the IMFINZI plus chemotherapy arm and 5.4 months (95% CI: 4.8, 6.2) in the chemotherapy alone arm. The investigator-assessed confirmed ORR was 68% (95% CI: 62%, 73%) in the IMFINZI plus chemotherapy arm and 58% (95% CI: 52%, 63%) in the chemotherapy alone arm.
In the exploratory subgroup analyses of OS based on the planned platinum chemotherapy received at cycle 1, the HR was 0.70 (95% CI 0.55, 0.89) in patients who received carboplatin, and the HR was 0.88 (95% CI 0.55, 1.41) in patients who received cisplatin.
Biliary Tract Cancer (BTC)
Locally Advanced or Metastatic BTC - TOPAZ-1
The efficacy of IMFINZI in combination with gemcitabine and cisplatin in patients with locally advanced or metastatic BTC was investigated in TOPAZ-1 (NCT03875235), a randomized, double-blind, placebo-controlled, multicenter study that enrolled 685 patients with histologically confirmed locally advanced unresectable or metastatic BTC who have not previously received systemic therapy. Patients with recurrent disease > 6 months after surgery and/or completion of adjuvant therapy were eligible. Patients had an ECOG Performance status of 0 and 1 and at least one target lesion by RECIST v1.1. Patients with ampullary carcinoma; active or prior documented autoimmune or inflammatory disorders; HIV infection or active infections, including tuberculosis or hepatitis C; current or prior use of immunosuppressive medication within 14 days before the first dose of IMFINZI were ineligible.
Randomization was stratified by disease status (recurrent vs. initially unresectable) and primary tumor location (intrahepatic cholangiocarcinoma [ICCA] vs. extrahepatic cholangiocarcinoma [ECCA] vs. gallbladder cancer [GBC]). Patients were randomized 1:1 to receive:
- IMFINZI 1,500 mg on Day 1+ gemcitabine 1,000 mg/m 2 and cisplatin 25 mg/m 2 on Days 1 and 8 of each 21-day cycle up to 8 cycles, followed by IMFINZI 1,500 mg every 4 weeks, or
- Placebo on Day 1+ gemcitabine 1,000 mg/m 2 and cisplatin 25 mg/m 2 on Days 1 and 8 of each 21-day cycle up to 8 cycles, followed by placebo every 4 weeks.
Treatment with IMFINZI or placebo continued until disease progression, or unacceptable toxicity. Treatment beyond disease progression was permitted if the patient was clinically stable and deriving clinical benefit as determined by the investigator.
The major efficacy outcome measure was overall survival (OS). Additional efficacy outcome measures were investigator-assessed progression-free survival (PFS), objective response rate (ORR) and duration of response (DoR). Tumor assessments were conducted every 6 weeks for the first 24 weeks after the date of randomization, and then every 8 weeks until confirmed objective disease progression.
The study population characteristics were: 50% male, median age of 64 years (range 20-85), 47% age 65 or older; 56% Asian, 37% White, 2% Black or African American, 0.1% American Indian or Alaskan Native, and 4% other; 51% had an ECOG PS of 1; primary tumor location was ICCA 56%, ECCA 18% and GBC 25%; 20% of patients had recurrent disease; 86% of patients had metastatic and 14% had locally advanced disease.
At a pre-specified interim analysis, the study demonstrated a statistically significant improvement in OS and PFS in patients randomized to IMFINZI in combination with chemotherapy compared to placebo in combination with chemotherapy. Table 31 summarizes the efficacy results for TOPAZ-1.
| Endpoint | IMFINZI with Gemcitabine and Cisplatin (N = 341) | Placebo with Gemcitabine and Cisplatin (N = 344) |
|---|---|---|
Overall Survival (OS) | ||
| 198 (58) | 226 (66) |
| 12.8 (11.1, 14) | 11.5 (10.1, 12.5) |
| 0.80 (0.66, 0.97) | |
| 0.021 | |
Progression-Free Survival (PFS) | ||
| 276 (81) | 297 (86) |
| 7.2 (6.7, 7.4) | 5.7 (5.6, 6.7) |
| 0.75 (0.63, 0.89) | |
| 0.001 | |
The investigator-assessed ORR was 27% (95% CI: 22% - 32%) in the IMFINZI plus chemotherapy arm and 19% (95% CI: 15%-23%) in the chemotherapy alone arm.
Figure 6. Kaplan-Meier Curves of OS in the TOPAZ-1 Study

Hepatocellular Carcinoma (HCC)
Unresectable HCC - HIMALAYA
The efficacy of IMFINZI in combination with tremelimumab-actl was evaluated in the HIMALAYA study (NCT03298451), a randomized (1:1:1), open-label, multicenter study in patients with confirmed uHCC who had not received prior systemic treatment for HCC. Patients were randomized to one of two investigational arms (IMFINZI plus tremelimumab-actl or IMFINZI) or sorafenib. Study treatment consisted of IMFINZI 1,500 mg in combination with tremelimumab-actl as a one-time single intravenous infusion of 300 mg on the same day, followed by IMFINZI every 4 weeks; IMFINZI 1,500 mg every 4 weeks; or sorafenib 400 mg given orally twice daily, until disease progression or unacceptable toxicity. The efficacy assessment of IMFINZI is based on patients randomized to the IMFINZI plus tremelimumab-actl arm versus the sorafenib arm. Randomization was stratified by macrovascular invasion (MVI) (yes or no), etiology of liver disease (hepatitis B virus vs. hepatitis C virus vs. others) and ECOG performance status (0 vs. 1).
The study enrolled patients with BCLC Stage C or B (not eligible for locoregional therapy). The study excluded patients with co-infection of viral hepatitis B and hepatitis C; active or prior documented gastrointestinal (GI) bleeding within 12 months; ascites requiring non-pharmacologic intervention within 6 months; hepatic encephalopathy within 12 months before the start of treatment; active or prior documented autoimmune or inflammatory disorders. Esophagogastroduodenoscopy was not mandated prior to enrollment but adequate endoscopic therapy, according to institutional standards, was required for patients with history of esophageal variceal bleeding or those assessed as high risk for esophageal variceal bleeding by the treating physician.
Study treatment was permitted beyond disease progression if the patient was clinically stable and deriving clinical benefit as determined by the investigator.
The major efficacy outcome measure was overall survival (OS) between the IMFINZI plus tremelimumab-actl arm versus the sorafenib arm. Additional efficacy outcomes were investigator-assessed progression-free survival (PFS), objective response rate (ORR) and duration of response (DoR) according to RECIST v1.1. Tumor assessments were conducted every 8 weeks for the first 12 months and then every 12 weeks thereafter.
The baseline demographics of the IMFINZI plus tremelimumab-actl and sorafenib arms were as follows: male (85%), age < 65 years (50%), median age of 65 years (range: 18 to 88 years), White (46%), Asian (49%), Black or African American (2%), Native Hawaiian or other Pacific Islander (0.1%), race Unknown (2%), Hispanic or Latino (5%), Not Hispanic or Latino (94%), ethnicity Unknown (1%), ECOG PS 0 (62%); Child-Pugh Class score A (99%), macrovascular invasion (26%), extrahepatic spread (53%), viral etiology; hepatitis B (31%), hepatitis C (27%), and uninfected (42%).
Efficacy results are presented in Table 32 and Figure 7.
| Endpoint | IMFINZI and Tremelimumab-actl (N = 393) | Sorafenib (N = 389) |
|---|---|---|
OS | ||
Number of deaths (%) | 262 (66.7) | 293 (75.3) |
Median OS (months) (95% CI) | 16.4 (14.2, 19.6) | 13.8 (12.3, 16.1) |
HR (95% CI) HR (IMFINZI and tremelimumab-actl vs. sorafenib) based on the stratified Cox proportional hazard model. | 0.78 (0.66, 0.92) | |
p-value Based on a stratified log-rank test. Based on a Lan-DeMets alpha spending function with O'Brien Fleming type boundary and the actual number of events observed, the boundary for declaring statistical significance for IMFINZI and tremelimumab-actl vs. sorafenib was 0.0398 (Lan and DeMets 1983). | 0.0035 | |
PFS | ||
Number of events (%) | 335 (85.2) | 327 (84.1) |
Median in (months) (95% CI) | 3.8 (3.7, 5.3) | 4.1 (3.7, 5.5) |
HR (95% CI) | 0.90 (0.77, 1.05) | |
ORR | ||
ORR % (95% CI) Confirmed complete response or partial response. Based on Clopper-Pearson method. | 20.1 (16.3, 24.4) | 5.1 (3.2, 7.8) |
Complete Response n (%) | 12 (3.1) | 0 |
Partial Response n (%) | 67 (17.0) | 20 (5.1) |
DoR | ||
Median DoR (months) (95% CI) | 22.3 (13.7, NR) | 18.4 (6.5, 26.0) |
% with duration ≥ 6 months | 82.3 | 78.9 |
% with duration ≥ 12 months | 65.8 | 63.2 |
CI=Confidence Interval, HR=Hazard Ratio, NR=Not Reached
Figure 7. Kaplan-Meier Curves of OS in the Himalaya Study

Endometrial cancer
Advanced or Recurrent dMMR Endometrial Cancer - DUO-E
IMFINZI was evaluated in combination with carboplatin and paclitaxel in DUO-E (NCT04269200), a randomized, multicenter, double-blind, placebo-controlled study in patients with advanced or recurrent endometrial cancer. The trial enrolled patients with newly diagnosed Stage III disease (with measurable disease per RECIST v1.1), or newly diagnosed Stage IV disease. The trial also enrolled patients with recurrent disease with a low potential for cure by radiation therapy or surgery. For patients with recurrent disease, prior chemotherapy was allowed only if it was administered in the adjuvant setting and at least 12 months had elapsed from the date of last dose of chemotherapy to the date of relapse. The trial included patients with epithelial endometrial carcinomas of all histologies, including carcinosarcomas. Patients with endometrial sarcoma were excluded, and patients who had active autoimmune disease or a medical condition that required immunosuppression were ineligible.
Randomization was stratified by tumor mismatch repair (MMR) status (proficient or deficient), disease status (recurrent or newly diagnosed), and geographic region (Asia or rest of the world). MMR status was assessed using an FDA-approved test.
Patients were randomized (1:1:1) to one of the following treatment arms:
- IMFINZI 1,120 mg in combination with carboplatin and paclitaxel every 3 weeks for a maximum of 6 cycles. Following completion of chemotherapy treatment, patients received IMFINZI 1,500 mg every 4 weeks as maintenance treatment until disease progression.
- Placebo in combination with carboplatin and paclitaxel every 3 weeks for a maximum of 6 cycles. Following completion of chemotherapy treatment, patients received placebo every 4 weeks as maintenance treatment until disease progression.
- An additional investigational combination regimen.
Treatment was continued until Response Evaluation Criteria in Solid Tumors (RECIST) v1.1-defined progression of disease or unacceptable toxicity. Assessment of tumor status was performed every 9 weeks for the first 18 weeks and every 12 weeks thereafter.
The major efficacy outcome measure was progression-free survival (PFS), determined by investigator assessment using RECIST 1.1. Additional efficacy outcome measures included overall response rate (ORR), duration of response (DOR) and overall survival (OS).
Among 95 patients with dMMR tumor, the baseline characteristics were median age of 63 years (range: 34 to 85); 47% age 65 or older; 62% White, 31% Asian, 2% Black or African American; 7% Hispanic or Latino, 1% American Indian or Alaska Native, and 4% other or not reported; ECOG PS of 0 (55%) or 1 (45%); 48% newly diagnosed (11% Stage III and 38% Stage IV) and 52% recurrent disease. The histologic subtypes were endometrioid (78%), mixed epithelial (6%), carcinosarcoma (5%), serous (4%), undifferentiated (1%), and other (5%).
While a statistically significant improvement in PFS was observed in the overall population for IMFINZI with carboplatin and paclitaxel compared to carboplatin and paclitaxel alone, based on an exploratory analysis by MMR status, the PFS improvement in the overall population was primarily attributed to patients with dMMR tumors.
Efficacy results for DUO-E are summarized in Table 33 and Figure 8 for patients with dMMR tumors. OS data in this subpopulation at the time of PFS analysis were immature with 26% of patients who died.
Endpoint | IMFINZI with Carboplatin and Paclitaxel N=46 | Carboplatin and Paclitaxel N=49 |
PFS Investigator assessed. | ||
| 15 (32.6) | 25 (51.0) |
| NR (NR, NR) | 7.0 (6.7, 14.8) |
| 0.42 (0.22, 0.80) | |
ORR | N=42 | N=42 |
| 71.4 (55.4, 84.3) | 40.5 (25.6, 56.7) |
| 12 (28.6) | 4 (9.5) |
| 18 (42.9) | 13 (31.0) |
DOR | ||
Median in months (range) | NR (2.4+, 26.9+) | 10.5 (2.1+, 25.2+) |
CI=Confidence Interval, HR=Hazard Ratio, NR=Not Reached, + = response ongoing at last assessment.
Figure 8. Kaplan-Meier curves of PFS for Patients with dMMR Tumors in the DUO-E Study

Muscle invasive bladder cancer (MIBC)
MIBC – NIAGARA Study
The efficacy of neoadjuvant IMFINZI in combination with gemcitabine and cisplatin followed by adjuvant IMFINZI as a single agent in patients with MIBC was evaluated in NIAGARA (NCT03732677), a randomized, open-label, multicenter study. The study randomized (1:1) 1,063 patients who were candidates for radical cystectomy and who had not received prior systemic chemotherapy or immune-mediated therapy for the treatment of NMIBC or MIBC. The study excluded patients with pure non-urothelial histology, any small cell histology and primary non-bladder (i.e., ureter, urethral, or renal pelvis) cancer of the urothelium.
Randomization was stratified by clinical tumor stage T2N0 vs. > T2N0 (including T2N1, T3, and T4a), renal function (creatinine clearance [CrCl] ≥ 60 mL/min vs. CrCl ≥ 40 mL/min to < 60 mL/min), and PD-L1 expression (high vs. low/negative). Patients received:
- Neoadjuvant IMFINZI 1,500 mg plus gemcitabine 1,000 mg/m 2 and cisplatin 70 mg/m 2 every 3 weeks for 4 cycles prior to surgery, followed by IMFINZI 1,500 mg every 4 weeks as a single agent adjuvant treatment for up to 8 cycles after surgery, or
- Neoadjuvant gemcitabine 1,000 mg/m 2 and cisplatin 70 mg/m 2 every 3 weeks for 4 cycles prior to surgery, without adjuvant treatment.
Patients with CrCl ≥ 40 mL/min to < 60 mL/min in both arms received split-dose cisplatin of 35 mg/m 2 on days 1 and 8 of each cycle.
A RECIST 1.1 tumor assessment was performed at baseline and upon completion of neoadjuvant therapy (prior to surgery). After surgery, tumor assessments were performed every 12 weeks for the first 24 months, then every 24 weeks for 36 months, and then every 52 weeks thereafter until progression, the end of study, or death.
The major efficacy outcome measure was EFS by BICR assessment. OS was an additional efficacy outcome measure.
Baseline demographics were: male (82%), median age 65 years (range: 32 to 84), age ≥65 years (53%), White (67%), Asian (28%), Black or African American (0.9%), other (0.8%), Hispanic or Latino (8%), and ECOG PS 0 (78%) vs. PS 1 (22%). Disease characteristics were: tumor stage T2N0 (40%) and >T2N0 (60%), regional lymph nodes N0 (95%) and N1 (5%), adequate renal function (81%) and borderline renal function (19%). The histologic subtypes included urothelial carcinoma (85%) and urothelial carcinoma with variant histology (15%).
In the overall population, 469 (88%) patients in the IMFINZI plus gemcitabine and cisplatin arm and 441 (83%) patients in the gemcitabine and cisplatin arm underwent radical cystectomy.
At a pre-specified interim analysis, the study demonstrated a statistically significant improvement in EFS and OS in the IMFINZI plus gemcitabine and cisplatin arm compared to the gemcitabine and cisplatin arm. The trial was not designed to isolate the effect of IMFINZI in each phase (neoadjuvant or adjuvant) of treatment. See Table 34 and Figures 9 and 10.
IMFINZI plus gemcitabine and cisplatin (N=533) | Gemcitabine and cisplatin (N = 530) | |
EFS EFS is defined as the time from randomization to the first recurrence of disease after radical cystectomy, the time of first documented progression in patients who are medically precluded from a radical cystectomy, or time of expected surgery in patients who refuse to undergo a radical cystectomy or failure to undergo a radical cystectomy in participants with residual disease, or the time of death due to any cause, whichever occurs first. | ||
Number of events (%) | 187. (35.1) | 246 (46.4) |
Median EFS (months) (95% CI) | NR (NR, NR) | 46.1 (32.2, NR) |
HR (95% CI) Based on stratified Cox proportional hazard model. | 0.68 (0.56, 0.82) | |
2-sided p-value Based on stratified log-rank test. , Alpha allocated at the interim analysis was 0.0412 for EFS and 0.0154 for OS. | <0.0001 | |
Overall Survival (OS) | ||
Number of events (%) | 136.(25.5) | 169 (31.9 |
Median OS (months) (95% CI) | NR (NR, NR) | NR (NR, NR) |
HR (95% CI) | 0.75 (0.59, 0.93) | |
2-sided p-value , | 0.0106 | |
CI = Confidence Interval, HR = Hazard Ratio, NR = Not Reached
Figure 9. Kaplan-Meier Curve of EFS
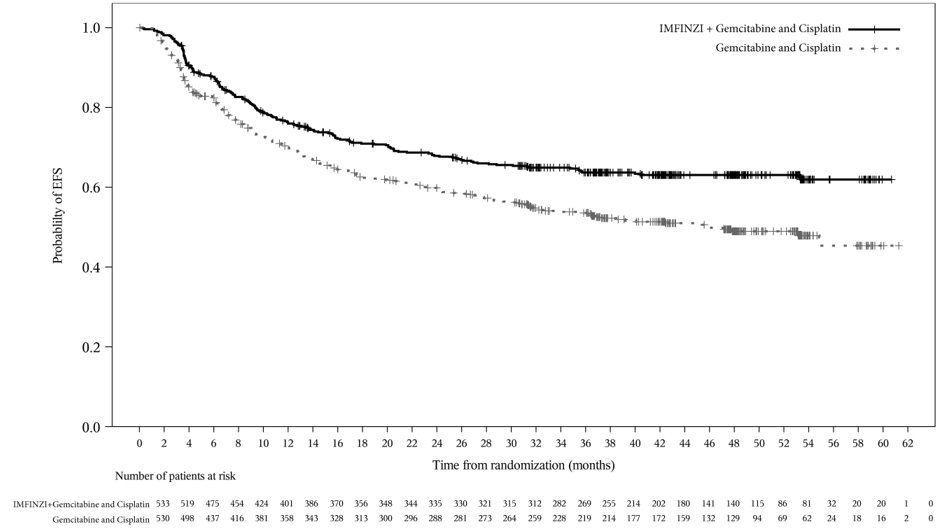
Figure 10. Kaplan-Meier Curve of OS
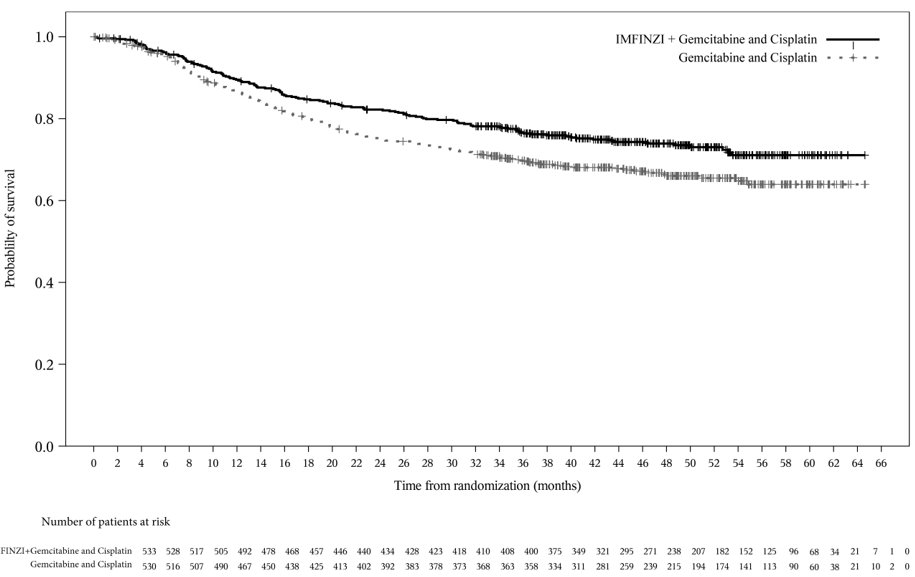
Gastric or gastroesophageal junction adenocarcinoma (GC/GEJC)
Neoadjuvant and Adjuvant Treatment of Resectable GC/GEJC – MATTERHORN Study
The efficacy of IMFINZI with FLOT as neoadjuvant and adjuvant treatment, followed by IMFINZI as a single agent, was investigated in MATTERHORN (NCT04592913), a randomized, double-blind, placebo-controlled, multicenter study conducted in 948 patients with previously untreated and resectable GC/GEJC (Stage II to Stage IVA [AJCC, 8th edition]). Eligible patients had no prior exposure to immune-mediated therapy and a WHO/ECOG performance status of 0 or 1. Patients with active or prior documented autoimmune or inflammatory disorders, or use of immunosuppressive medication within 14 days of the first dose of durvalumab were ineligible.
Randomization was stratified by geographic region (Asia vs. non-Asia), clinical lymph node status (positive vs. negative), and PD-L1 expression status (TAP < 1% vs. TAP ≥ 1%).
Patients were randomized in a 1:1 ratio to one of the following treatment arms. Crossover between the study arms was not permitted.
- IMFINZI 1,500 mg on Day 1 + FLOT on Days 1 and 15 of every 4-week cycle for 2 cycles in the neoadjuvant phase and 2 cycles in the adjuvant phase followed by IMFINZI 1,500 mg on Day 1 every 4 weeks for up to 10 additional cycles for a total of 12 cycles (1 dose per cycle)
- Placebo on Day 1 + FLOT on Days 1 and 15 of every 4-week cycle for 2 cycles in the neoadjuvant phase and 2 cycles in the adjuvant phase followed by placebo on Day 1 every 4 weeks for up to 10 additional cycles for a total of 12 cycles (1 dose per cycle)
Tumor assessment per RECIST was performed prior to the start of neoadjuvant therapy, prior to surgery, before starting adjuvant treatment and then every 12 weeks for 2 years and every 24 weeks until RECIST 1.1 defined radiological progression, consent withdrawal, or death.
The major efficacy outcome measure of the study was EFS by BICR assessment. EFS was defined as the earliest of one of the following: disease progression according to RECIST 1.1 that precluded surgery or that required non-protocol therapy during the neoadjuvant treatment period; progression (after R1/R2 resection) or recurrence (after R0 resection), according to RECIST 1.1, during the adjuvant treatment period; non-RECIST progression (according to investigator assessment or confirmed by local pathology testing) that precluded surgery or that required non-protocol therapy during the neoadjuvant treatment period, or that was discovered during surgery; progression or recurrence confirmed by local pathology testing after surgery; or death.
Additional efficacy outcome measures were OS and pCR rate by blinded central pathology review. The trial was not designed to isolate the effect of IMFINZI in each phase (neoadjuvant or adjuvant) of treatment.
The demographics were as follows: median age 62 years (range: 26 to 84), age ≥65 years (41%), male (72%), White (68%), Asian (20%), American Indian or Alaska Native (4%), Black or African American (1.1%), other race (1.7%), Hispanic or Latino (20%), and WHO/ECOG PS 0 (74%). Disease characteristics were as follows: Stage II (29%), Stage III (62%), Stage IVA (9%), gastric (68%), gastroesophageal junction (32%), Siewert type 1 (10%), Siewert type 2 (15%), Siewert type 3 (7%), intestinal type (51%), diffuse type (26%), indeterminate type (23%), clinical lymph node status positive (70%), clinical lymph node status negative (29%), PD-L1 expression status TAP ≥1% (90%), and PD-L1 expression status TAP <1% (10%).
There were 412 (87%) patients in the IMFINZI arm who completed curative intent surgery compared to 400 (84%) patients in the placebo arm. Surgery was delayed (defined as surgery attempt >8 weeks from last dose of neoadjuvant therapy) in 48 (10%) patients in the IMFINZI arm and 51 (11%) patients in the placebo arm. In the IMFINZI arm, 248 (52%) and 291 (61%) patients completed the two cycles of adjuvant IMFINZI and two cycles of adjuvant FLOT, respectively. In the placebo arm, 245 (52%) and 303 (64%) patients completed the two cycles of adjuvant placebo and two cycles of adjuvant FLOT, respectively. There were 344 (73%) patients in the IMFINZI arm who initiated adjuvant IMFINZI monotherapy compared to 332 (70%) patients in the placebo arm.
The study demonstrated a statistically significant improvement in EFS in the IMFINZI arm compared to the placebo arm. See Table 35, Figure 11, and Figure 12.
| IMFINZI + FLOT chemotherapy (N = 474) | Placebo + FLOT chemotherapy (N = 474) | |
|---|---|---|
Event Free Survival (EFS) | ||
Number of events, n (%) | 167 (35) | 218 (46) |
Median EFS (months) (95% CI) | NR (40.7, NE) | 32.8 (27.9, NE) |
HR (95% CI) | 0.71 (0.58, 0.86) | |
p-value | <0.001 | |
Overall Survival (OS) | ||
Number of events (%) | 160 (33.8) | 192 (40.5) |
Median OS (months) (95% CI) | NR (NE, NE) | NR (NE, NE) |
HR (95% CI) | 0.78 (0.63, 0.96) | |
p-value | 0.021 | |
pCR Results for EFS are based on a pre-specified interim analysis (DCO: 20 December 2024), results for OS are based on a pre-specified final analysis (DCO: 01 September 2025), and results for pCR are based on a pre-specified final analysis (DCO: 01 February 2023). | ||
Number of patients with response | 91 | 34 |
Response rate, (%) (95% CI) | 19.2 (15.7, 23.0) | 7.2 (5.0, 9.9) |
p-value | <0.001 | |
CI=Confidence Interval, HR=Hazard Ratio, NE=Not estimable, NR=Not reached
Figure 11. Kaplan-Meier Curve of EFS
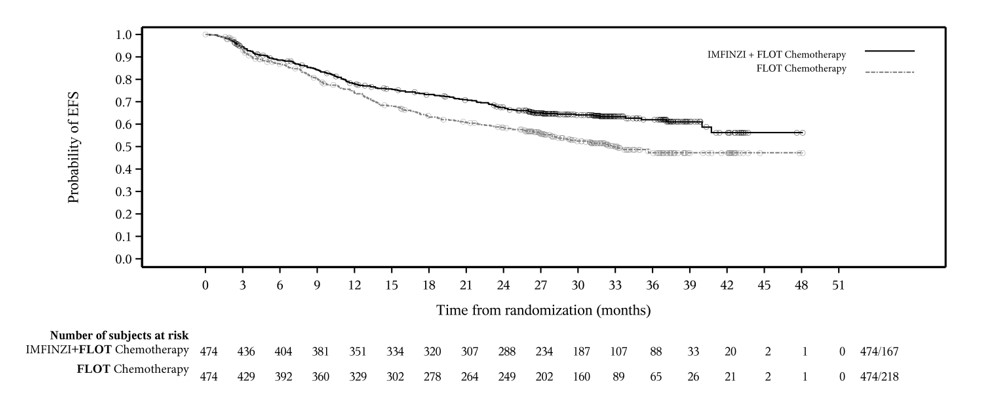
Figure 12. Kaplan-Meier Curve of OS
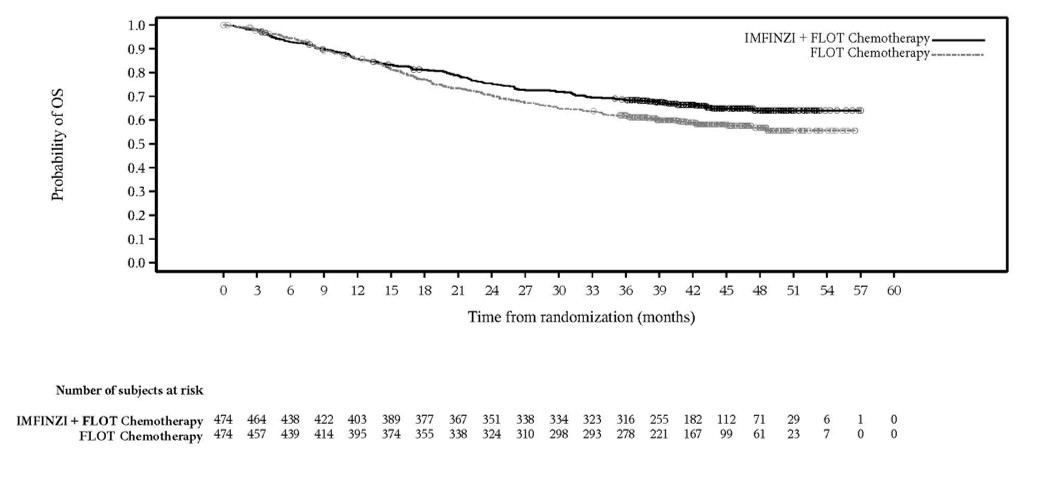
HOW SUPPLIED/STORAGE AND HANDLING
IMFINZI (durvalumab) Injection is a clear to opalescent, colorless to slightly yellow solution supplied in a carton containing one single-dose vial either as:
- 500 mg/10 mL (50 mg/mL) (NDC 0310-4611-50)
- 120 mg/2.4 mL (50 mg/mL) (NDC 0310-4500-12)
Store in a refrigerator at 2°C to 8°C (36°F to 46°F) in original carton to protect from light.
Do not freeze. Do not shake.
Mechanism of Action
Expression of programmed cell death ligand-1 (PD-L1) can be induced by inflammatory signals (e.g., IFN-gamma) and can be expressed on both tumor cells and tumor-associated immune cells in the tumor microenvironment. PD-L1 blocks T-cell function and activation through interaction with PD-1 and CD80 (B7.1). By binding to its receptors, PD-L1 reduces cytotoxic T-cell activity, proliferation, and cytokine production.
Durvalumab is a human immunoglobulin G1 kappa (IgG1κ) monoclonal antibody that binds to PD-L1 and blocks the interaction of PD-L1 with PD-1 and CD80 (B7.1). Blockade of PD-L1/PD-1 and PD-L1/CD80 interactions releases the inhibition of immune responses, without inducing antibody dependent cell-mediated cytotoxicity (ADCC).
PD-L1 blockade with durvalumab led to increased T-cell activation in vitro and decreased tumor size in co-engrafted human tumor and immune cell xenograft mouse models.