Iopamidol - Iopamidol injection, Solution prescribing information
WARNING: RISKS ASSOCIATED WITH INTRATHECAL ADMINISTRATION
Intrathecal administration, even if inadvertent, can cause death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema [see Warnings and Precautions (5.1 )] . Iopamidol Injection, USP is for intra-arterial or intravenous use only [see Dosage and Administration (2.1 )] .
INDICATIONS AND USAGE
Iopamidol Injection, USP is a radiographic contrast agent indicated for:
Intra-arterial Procedures • (1.1 )
- Cerebral arteriography in adults
- Peripheral arteriography in adults
- Selective visceral arteriography and aortography in adults
- Coronary arteriography and cardiac ventriculography in adults
- Angiocardiography in pediatric patients
Intravenous Procedures • (1.2 )
- Excretory urography in adults and pediatric patients
- Computed tomography (CT) of head and body in adults and pediatric patients
- Peripheral venography in adults
• Specific concentrations are recommended for each type of imaging procedure. (2.2 , 2.3 , 2.4 )
Intra-arterial Procedures•
- Cerebral arteriography in adults
- Peripheral arteriography in adults
- Selective visceral arteriography and aortography in adults
- Coronary arteriography and cardiac ventriculography in adults
- Angiocardiography in pediatric patients
Intravenous Procedures•
- Excretory urography in adults and pediatric patients
- Computed tomography (CT) of head and body in adults and pediatric patients
- Peripheral venography in adults
• Specific concentrations of Iopamidol Injection, USP are recommended for each type of imaging procedure [see Dosage and Administration (2.2 , 2.3 , 2.4 )].
DOSAGE AND ADMINISTRATION
- Individualize the volume and concentration according to the specific dosing tables accounting for factors such as age, body weight, size of the vessel, and the rate of blood flow within the vessel. (2.2 , 2.3 , 2.4 )
- See full prescribing information for important dosage and administration information. (2.1 )
Important Dosing and Administration Information
- Iopamidol Injection, USP is for intra-arterial or intravenous use only and must not be administered intrathecally [see Warnings and Precautions (5.1 )] .
- Specific concentrations of Iopamidol Injection, USP are recommended for each type of imaging procedure [see Dosage and Administration (2.2 , 2.3 , 2.4 )] .
- Individualize the volume, concentration, and injection rate of iopamidol injection according to the specific dosing tables [see Dosage and Administration (2.2 , 2.3 , 2.4 )] . Consider factors such as: age, body weight, blood vessel size and blood flow rate, anticipated pathology and degree and extent of opacification required, structures or area to be examined, concomitant medical conditions, imaging equipment, and technique to be employed.
- Hydrate patients before and after Iopamidol Injection, USP administration [see Warnings and Precautions (5.3 )] .
- Use aseptic technique for all handling and administration of Iopamidol Injection, USP.
- Iopamidol Injection, USP may be administered at either body temperature (37°C, 98.6°F) or room temperature (20°C to 25°C, 68°F to 77°F).
- Visually inspect Iopamidol Injection, USP for particulate matter or discoloration before administration. Do not administer Iopamidol Injection, USP if particulate matter or discolorations are observed.
- Do not mix Iopamidol Injection, USP with other drugs or inject in intravenous lines containing other drugs or total nutritional admixtures.
- Iopamidol Injection, USP single-dose containers are intended for one procedure only. Discard any unused portion.
Recommended Dosage for Intra-arterial Procedures in Adults
The recommended doses for intra-arterial procedures in adults are shown in Table 1.
Table 1: Recommended Concentrations and Volumes of Iopamidol Injection, USP for Intra-arterial Procedures in Adults
Imaging Procedure | Concentration (mg Iodine/mL) | Volume to Administer per Single Injection for Selected Injection Sites | Maximum Cumulative Total Dose |
Cerebral Arteriography | 300 | 8 mL to 12 mL by carotid puncture or transfemoral catheterization | 90 mL |
Peripheral Arteriography | 300 | • 5 mL to 40 mL into the femoral artery or subclavian artery • 25 mL to 50 mL into the aorta for a distal runoff | 250 mL |
Selective Visceral Arteriography and Aortography | 370 | • Up to 10 mL for the renal arteries • Up to 50 mL into the larger vessels such as the aorta or celiac artery | 225 mL |
Coronary Arteriography and Cardiac Ventriculography | 370 | • 2 mL to 10 mL for selective coronary artery injection • 25 mL to 50 mL for cardiac ventriculography or for nonselective opacification of multiple coronary arteries following injection at the aortic root | 200 mL |
Recommended Dosage for Intravenous Procedures in Adults
The recommended doses for intra-arterial procedures in adults are shown in Table 2.
Table 2: Recommended Concentrations and Volumes of Iopamidol Injection, USP for Intravenous Procedures in Adults
Imaging Procedure | Concentration (mg Iodine/mL) | Volume to Administer |
Excretory Urography | 250 300 370 | 50 mL to 100 mL by rapid injection 50 mL by rapid injection 40 mL by rapid injection |
CT of the Head | 250 300 | 130 mL to 240 mL 100 mL to 200 mL |
CT of Body | 250 300 370 | 130 mL to 240 mL by rapid infusion or bolus injection 100 mL to 200 mL by rapid infusion or bolus injection 80 mL to 160 mL by rapid infusion or bolus injection |
Peripheral Venography | 200 | 25 mL to 150 mL per lower extremity; the maximum total dose is 350 mL |
Recommended Dosage in Pediatric Patients
The recommended doses in pediatric patients are shown in Table 3.
Table 3: Recommended Concentrations and Volumes per Body Weight of Iopamidol Injection, USP for Intra-arterial and Intravenous Procedures in Pediatric Patients
Imaging Procedure | Concentration (mg Iodine/mL) | Volume per Body Weight to Administer | Maximum Dose |
Intra-arterial Procedures | |||
Angiocardiography | 370 | 0.5 mL/kg to 2 mL/kg per single injection | Maximum Cumulative Dose by Weight • Neonates: 5 mL/kg • Aged 4 weeks and older: 8 mL/kg • Do not exceed maximum cumulative doses by age below. Maximum Cumulative Dose by Age • <2 years 40 mL • 2 years to 4 years 50 mL • 5 years to 9 years 100 mL • 10 years to 18 years 125 mL |
Intravenous Procedures | |||
Excretory Urography | 250 | 1.2 mL/kg to 3.6 mL/kg | 120 mL |
300 | 1 mL/kg to 3 mL/kg | 100 mL | |
CT of the Head and Body | 250 | 1.2 mL/kg to 3.6 mL/kg | 120 mL |
300 | 1 mL/kg to 3 mL/kg | 100 mL | |
DOSAGE FORMS AND STRENGTHS
Injection: Clear, colorless to pale yellow solution available in the following concentrations of iodine:
Concentration (mg Iodine/mL) | Package Size | Package Type |
200 | 50 mL | Single-Dose Vial |
250 | 50 mL | Single-Dose Vial |
300 | 30 mL, 50 mL, 100 mL | Single-Dose Vial |
370 | 50 mL, 75 mL, and 100 mL | Single-Dose Vial |
USE IN SPECIFIC POPULATIONS
Lactation: A lactating woman may pump and discard breast milk for 10 hours after iopamidol injection administration. (8.2 )
Pregnancy
Risk Summary
Available data from published literature and postmarketing cases from decades of use with iopamidol during pregnancy have not identified a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Iopamidol crosses the placenta and reaches fetal tissues in small amounts (see Data). In animal reproduction studies, no adverse developmental outcomes were observed with intravenous administration of iopamidol to pregnant rats and rabbits during organogenesis at doses up to 2.7 and 1.4 times, respectively, the maximum recommended human dose (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Human Data
Literature reports show that intravenously administered iopamidol crosses the placenta and is visualized in the digestive tract of exposed infants after birth.
Animal Data
Iopamidol did not affect fetal development and did not induce teratogenic changes in the offspring in either rats or rabbits at the following dose levels tested: 600 mg, 1,500 mg, or 4,000 mg iodine/kg in rats, administered intravenously once a day during days 6 through 15 of pregnancy; 300 mg, 800 mg, or 2,000 mg iodine/kg in rabbits, administered intravenously once a day during days 6 through 18 of pregnancy.
Lactation
Risk Summary
There are no data on the presence of iopamidol in human milk, the effects on the breastfed infant, or the effects on milk production. Iodinated contrast agents are present unchanged in human milk in very low amounts, with poor absorption from the gastrointestinal tract of a breastfed infant. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for iopamidol injection and any potential adverse effects on the breastfed infant from iopamidol injection or from the underlying maternal condition.
Clinical Considerations
Interruption of breastfeeding after exposure to iodinated contrast agents is not necessary because the potential exposure of the breastfed infant to iodine is small. However, a lactating woman may consider interrupting breastfeeding and pumping and discarding breast milk for 10 hours (approximately 5 half-lives) after iopamidol injection administration in order to minimize drug exposure to a breastfed infant.
Pediatric Use
The safety and effectiveness of iopamidol injection have been established in pediatric patients for angiocardiography, excretory urography, and contrast computed tomography (head and body).
Pediatric patients at higher risk of experiencing adverse reactions during and after contrast medium administration may include those having asthma, sensitivity to medication or allergens, cyanotic heart disease, congestive heart failure, or serum creatinine greater than 1.5 mg/dL, or those less than 12 months of age.
Thyroid function tests indicative of thyroid dysfunction, characterized by hypothyroidism or transient thyroid suppression have been reported following iodinated contrast media administration in pediatric patients, including term and preterm neonates; some patients were treated for hypothyroidism. After exposure to iodinated contrast media, individualize thyroid function monitoring in pediatric patients 0 years to 3 years of age based on underlying risk factors, especially in term and preterm neonates [see Warning and Precautions (5.8 ) and Adverse Reactions (6.2 )] .
The safety and effectiveness of iopamidol injection for cerebral, peripheral, and selective visceral arteriography, aortography, coronary arteriography, cardiac ventriculography, and peripheral venography have not been established in pediatric patients.
Geriatric Use
Iopamidol is excreted by the kidney, and the risk of adverse reactions to iopamidol injection may be greater in patients with renal impairment. Because patients 65 years of age and older are more likely to have renal impairment, care should be taken in dose selection, and it may be useful to monitor renal function [see Warnings and Precautions (5.3 ) and Use in Specific Populations (8.6 )] .
Renal Impairment
The clearance of iopamidol decreases with increasing degree of renal impairment and results in delayed opacification of the urinary system. In addition, preexisting renal impairment increases the risk for acute kidney injury [see Warnings and Precautions (5.3 )]. Iopamidol can be removed by dialysis.
CONTRAINDICATIONS
None.
WARNINGS AND PRECAUTIONS
- Hypersensitivity Reactions: Life-threatening or fatal reactions can occur. Always have emergency resuscitation equipment and trained personnel available. (5.2 )
- Acute Kidney Injury: Acute injury including renal failure can occur. Use the lowest dose and maintain adequate hydration to minimize risk. (5.3 )
- Cardiovascular Adverse Reactions: Hemodynamic disturbances including shock and cardiac arrest may occur during or after iopamidol injection administration. (5.4 )
- Thyroid Dysfunction in Pediatric Patients 0 Years to 3 Years of Age: Individualize thyroid function monitoring based on risk factors such as prematurity. (5.8 )
Risks Associated with Intrathecal Administration
Intrathecal administration, even if inadvertent, can cause death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema. Iopamidol injection is for intra-arterial or intravenous use only and must not be administered intrathecally [see Dosage and Administration (2.1 )].
Hypersensitivity Reactions
Iopamidol injection can cause life-threatening or fatal hypersensitivity reactions including anaphylaxis. Manifestations include respiratory arrest, laryngospasm, bronchospasm, angioedema, and shock [see Adverse Reactions (6.2 )] . Most severe reactions develop shortly after the start of injection (e.g., within 1 to 3 minutes), but delayed reactions can also occur. There is increased risk of hypersensitivity reactions in patients with a history of previous reactions to contrast agents, and allergic disorders (i.e., bronchial asthma, allergic rhinitis, and food allergies) or other hypersensitivities.
Premedication with antihistamines or corticosteroids to avoid or minimize possible allergic reactions does not prevent serious life-threatening reactions but may reduce both their incidence and severity. Obtain a history of allergy, hypersensitivity, or hypersensitivity reactions to iodinated contrast agents and always have emergency resuscitation equipment and trained personnel available prior to iopamidol injection administration. Monitor all patients for hypersensitivity reactions.
Acute Kidney Injury
Acute kidney injury, including renal failure, may occur after iopamidol injection administration. Risk factors include: pre-existing renal insufficiency, dehydration, diabetes mellitus, congestive heart failure, advanced vascular disease, elderly age, concomitant use of nephrotoxic or diuretic medications, multiple myeloma or other paraproteinemias, and repetitive or large doses of iopamidol injection.
Use the lowest necessary dose of iopamidol injection in patients with renal impairment. Adequately hydrate patients prior to and following iopamidol injection administration. Do not use laxatives, diuretics, or preparatory dehydration prior to iopamidol injection administration.
Cardiovascular Adverse Reactions
Iopamidol injection increases the circulatory osmotic load and may induce acute or delayed hemodynamic disturbances in patients with congestive heart failure, severely impaired renal function, combined renal and hepatic disease, and combined renal and cardiac disease, particularly when repetitive or large doses are administered. Fatal cardiovascular reactions have occurred mostly within 10 minutes of iopamidol injection; the main feature was cardiac arrest with cardiovascular disease as the main underlying factor. Hypotensive collapse and shock have occurred. Cardiac decompensation, serious arrhythmias, and myocardial ischemia or infarction can occur during coronary arteriography and ventriculography.
The administration of iopamidol injection may cause pulmonary edema in patients with heart failure. Based upon published reports, deaths associated with the administration of iodinated contrast agents range from 6.6 per 1 million (0.00066 percent) to 1 in 10,000 patients (0.01 percent). Use the lowest necessary dose of iopamidol injection in patients with congestive heart failure and always have emergency resuscitation equipment and trained personnel available. Monitor all patients for severe cardiovascular reactions.
Thromboembolic Events
Serious, in some cases fatal, thromboembolic events, including myocardial infarction and stroke, can occur during angiographic procedures. During these procedures, increased thrombosis and activation of the complement system occurs. Risk factors for developing thromboembolic events include: length of procedure, catheter and syringe material, underlying disease state, and concomitant medications.
To minimize thromboembolic events, use meticulous angiographic techniques and minimize the length of the procedure. Avoid blood remaining in contact with syringes containing iodinated contrast agents, which increases risk of clotting. Avoid angiocardiography in patients with homocystinuria because of the risk of inducing thrombosis and embolism.
Extravasation and Injection Site Reactions
Extravasation can occur with iopamidol injection administration, particularly in patients with severe arterial or venous disease. Inflammation, blistering, skin necrosis, and compartment syndrome have been reported following extravasation. In addition, injection site reactions such as pain and swelling at the injection site can also occur [see Adverse Reactions (6.2 )] . Ensure intravascular placement of catheters prior to injection. Monitor patients for extravasation and advise patients to seek medical care for progression of symptoms.
Thyroid Storm in Patients with Hyperthyroidism
Thyroid storm has occurred after the intravascular use of iodinated agents in patients with hyperthyroidism or with an autonomously functioning thyroid nodule. Evaluate the risk in such patients before use of iopamidol injection
Thyroid Dysfunction in Pediatric Patients 0 Years to 3 Years of Age
Thyroid dysfunction characterized by hypothyroidism or transient thyroid suppression has been reported after both single exposure and multiple exposures to iodinated contrast agents in pediatric patients 0 years to 3 years of age.
Younger age, very low birth weight, prematurity, underlying medical conditions affecting thyroid function, admission to neonatal or pediatric intensive care units, and congenital cardiac conditions are associated with an increased risk of hypothyroidism after iodinated contrast agent exposure. Pediatric patients with congenital cardiac conditions may be at greatest risk given that they often require high doses of contrast during invasive cardiac procedures.
An underactive thyroid during early life may be harmful for cognitive and neurological development and may require thyroid hormone replacement therapy. After exposure to iodinated contrast agents, individualize thyroid function monitoring based on underlying risk factors, especially in term and preterm neonates.
Hypertensive Crisis in Patients with Pheochromocytoma
Hypertensive crisis in patients with pheochromocytoma has occurred with iodinated contrast agents. Closely monitor patients when administering iopamidol injection if pheochromocytoma or catecholamine-secreting paragangliomas are suspected. Inject the minimum amount of iopamidol injection necessary and have measures for treatment of hypertensive crisis readily available.
Sickle Cell Crisis in Patients with Sickle Cell Disease
Iodinated contrast agents can promote sickling in individuals who are homozygous for sickle cell disease. Hydrate patients prior to and following iopamidol injection administration and use only if the necessary imaging information cannot be obtained with alternative imaging modalities.
Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions (SCAR) may develop from 1 hour to several weeks after intravascular contrast agent administration. These reactions include Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), acute generalized exanthematous pustulosis (AGEP), and drug reaction with eosinophilia and systemic symptoms (DRESS). Reaction severity may increase and time to onset may decrease with repeat administration of a contrast agent; prophylactic medications may not prevent or mitigate severe cutaneous adverse reactions. Avoid administering iopamidol injection to patients with a history of a severe cutaneous adverse reaction to iopamidol injection.
Interference with Laboratory Tests
Iopamidol injection can interfere with protein-bound iodine test [see Drug Interactions (7.2 )] .
ADVERSE REACTIONS
The following adverse reactions are described in greater detail in other sections:
- Risks Associated with Intrathecal Administration [see Warnings and Precautions (5.1 )]
- Hypersensitivity Reactions [see Warnings and Precautions (5.2 )]
- Acute Kidney Injury [see Warnings and Precautions (5.3 )]
- Cardiovascular Adverse Reactions [see Warnings and Precautions (5.4 )]
- Thromboembolic Events [see Warnings and Precautions (5.5 )]
- Extravasation and Injection Site Reactions [see Warnings and Precautions (5.6 )]
- Thyroid Dysfunction in Pediatric Patients 0 to 3 Years of Age [see Warnings and Precautions (5.8 )]
- Severe Cutaneous Adverse Reactions [see Warnings and Precautions (5.11 )]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions in Adults
The safety of iopamidol injection was evaluated in 2,246 adult patients receiving iopamidol injection by intra-arterial or intravenous route in clinical studies. Table 4 shows the common adverse reactions (>1%).
Table 4: Adverse Reactions Reported in >1% of Patients Receiving Intra-arterial or Intravenous Injection of Iopamidol in Clinical Studies
Adverse Reaction | Iopamidol Injection (N=2,246) % |
Pain | 2.8 |
Hot flashes | 1.5 |
Burning sensation | 1.4 |
Nausea | 1.2 |
Warmth | 1.1 |
The following adverse reactions occurred in ≤ 1% of patients receiving intra-arterial or intravenous injection of iopamidol injection:
Cardiovascular disorders : tachycardia, hypotension, hypertension, myocardial ischemia, circulatory collapse, S-T segment depression, bigeminy, extrasystoles, ventricular fibrillation, angina pectoris, bradycardia, transient ischemic attack, thrombophlebitis
Gastrointestinal disorders : vomiting, anorexia
General disorders : headache, fever, chills, excessive sweating, back spasm
Nervous system disorders : vasovagal reaction, tingling in arms, grimace, faintness
Renal and urinary disorders : urinary retention
Respiratory : throat constriction, dyspnea, pulmonary edema
Skin and subcutaneous tissues : rash, urticaria, pruritus, flushing
Special senses : taste alterations, nasal congestion, visual disturbances
Adverse Reactions in Pediatric Patients
In a clinical trial with 76 pediatric patients undergoing angiocardiography, two adverse reactions (2.6%) were reported: worsening cyanosis and worsening peripheral perfusion.
Postmarketing Experience
The following adverse reactions have been identified during post approval use of iopamidol injection. Because the reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or to establish a causal relationship to drug exposure.
Blood and lymphatic system disorders : thrombocytopenia
Cardiovascular disorders : cardiopulmonary arrest, cardiac decompensation, arrhythmias, myocardial infarction, shock, electrocardiographic changes (e.g., increased QTc, increased R-R, increased T-wave amplitude), decreased systolic pressure, deep vein thrombosis, arterial spasms, vasodilation, chest pain, pallor
Endocrine disorders : hyperthyroidism, hypothyroidism
Eye disorders : lacrimation increased, conjunctivitis, eye pruritus, transient blindness, visual disturbance, photophobia
Gastrointestinal disorders : retching, abdominal pain, salivary hypersecretion, salivary gland enlargement
General disorders and administration site conditions : injection site pain, malaise
Immune system disorders : anaphylaxis characterized by cardiovascular, respiratory, and cutaneous manifestations (e.g., chest tightness, laryngeal edema, periorbital edema, facial edema); delayed hypersensitivity reactions including generalized maculopapular rash,erythema, pruritus, localized blistering, skin peeling
Musculoskeletal disorders : compartment syndrome following extravasation, muscle spasm, musculoskeletal pain, muscular weakness
Nervous system disorders : coma, seizure, tremors, syncope, depressed level of consciousness or loss of consciousness, encephalopathy
Psychiatric disorders : confusional state
Respiratory system disorders : respiratory arrest, respiratory failure, acute respiratory distress syndrome, respiratory distress, apnea, asthma, sneezing, choking, laryngeal edema, bronchospasm, rhinitis
Skin and subcutaneous tissue disorders : Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), acute generalized exanthematous pustulosis (AGEP), erythema multiforme and drug reaction with eosinophilia and systemic symptoms (DRESS), skin necrosis, face edema
DRUG INTERACTIONS
Drug-Drug Interactions
Metformin
In patients with renal impairment, metformin can cause lactic acidosis. Iodinated contrast agents appear to increase the risk of metformin-induced lactic acidosis, possibly as a result of worsening renal function.
Stop metformin at the time of, or prior to, iopamidol injection administration in patients with an eGFR between 30 and 60 mL/min/1.73 m 2 ; in patients with a history of hepatic impairment, alcoholism, or heart failure; or in patients who will be administered intra-arterial iodinated contrast agents. Re-evaluate eGFR 48 hours after the imaging procedure and reinstitute metformin use only after renal function is stable.
Radioactive Iodine
Administration of iopamidol injection may interfere with thyroid uptake of radioactive iodine (I-131 and I-123) and decrease therapeutic and diagnostic efficacy. Avoid thyroid therapy or testing for up to 6 weeks post iopamidol injection
Drug-Laboratory Test Interactions
Protein-Bound Iodine Test
Iodinated contrast agents, including iopamidol injection, will temporarily increase protein-bound iodine in blood. Avoid protein-bound iodine test for at least 16 days following administration of iopamidol injection . However, thyroid function tests that do not depend on iodine estimations, e.g., triiodothyronine (T 3 ) resin uptake and total or free thyroxine (T 4 ) assays, are not affected.
DESCRIPTION
Iopamidol Injection, USP is a radiographic contrast agent for intra-arterial or intravenous use.
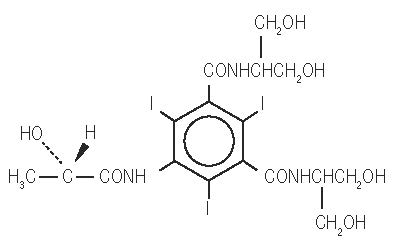
Iopamidol is designated chemically as (S)-N,N’-bis[2-hydroxy-1-(hydroxymethyl)-ethyl]-2,4,6-triiodo-5-lactamidoisophthalamide with a molecular weight of 777.09, an empirical formula of C 17 H 22 I 3 N 3 O 8 , and the following structural formula:
Iopamidol Injection, USP is a sterile, clear, colorless to pale yellow solution available in four concentrations of iodine:
- Iopamidol Injection, USP, 200 mg iodine/mL: Each mL contains 408 mg iopamidol (providing 200 mg organically bound iodine) and the following inactive ingredients: 0.26 mg edetate calcium disodium (providing 0.029 mg (0.001 mEq) sodium) and 1 mg tromethamine.
- Iopamidol Injection, USP, 250 mg iodine/mL: Each mL contains 510 mg iopamidol (providing 250 mg organically bound iodine) and the following inactive ingredients: 0.33 mg edetate calcium disodium (providing 0.036 mg (0.002 mEq) sodium) and 1 mg tromethamine.
- Iopamidol Injection, USP, 300 mg iodine/mL: Each mL contains 612 mg iopamidol (providing 300 mg organically bound iodine) and the following inactive ingredients: 0.39 mg edetate calcium disodium (providing 0.043 mg (0.002 mEq) sodium) and 1 mg tromethamine.
- Iopamidol Injection, USP, 370 mg iodine/mL: Each mL contains 755 mg iopamidol (providing 370 mg organically bound iodine) and the following inactive ingredients: 0.48 mg edetate calcium disodium (providing 0.053 mg (0.002 mEq) sodium) and 1 mg tromethamine.
The pH of Iopamidol Injection, USP has been adjusted to 6.5 to 7.5 with hydrochloric acid and/or sodium hydroxide.
Physicochemical characteristics are shown in Table 5. Iopamidol Injection, USP is hypertonic as compared to plasma and cerebrospinal fluid (approximately 285 and 301 mOsm/kg water, respectively).
Table 5: Physicochemical Characteristics of Iopamidol Injection, USP
Concentration (mg Iodine/mL) | 200 | 250 | 300 | 370 |
Osmolality @ 37°C (mOsm/kg water) | 413 | 524 | 616 | 796 |
Viscosity (cP) @ 37°C | 2.0 | 3.0 | 4.7 | 9.4 |
Viscosity (cP) @ 20°C | 3.3 | 5.1 | 8.8 | 20.9 |
Specific Gravity @ 37°C | 1.227 | 1.281 | 1.339 | 1.405 |
CLINICAL PHARMACOLOGY
Mechanism of Action
Intravascular injection of iopamidol opacifies those vessels where the contrast agent is present, permitting radiographic visualization through attenuation of photons.
In imaging of the body, iodinated contrast agents diffuse from the vessels into the extravascular space. In normal brain with an intact blood-brain barrier, contrast does not diffuse into the extravascular space. In patients with a disrupted blood-brain barrier, contrast agent accumulates in the extravascular space in the region of disruption.
Pharmacodynamics
Following administration of iopamidol injection, the degree of enhancement is related to the iodine concentration in the tissue of interest. However, the exposure-response relationships and time course of pharmacodynamic response of iopamidol have not been fully characterized.
Pharmacokinetics
Distribution
Plasma concentrations of iodine fall within 5 to 10 minutes due to distribution into the vascular and extracellular fluid compartments. Equilibration with the extracellular compartments is reached in about 10 minutes.
The apparent volume of distribution suggests that iopamidol is distributed evenly between blood and extracellular fluid. Iopamidol may be visualized in the renal parenchyma within 30 to 60 seconds following rapid intravenous administration. Iopamidol did not bind to serum or plasma proteins at 1 hour after administration.
Elimination
The plasma half-life is approximately 2 hours; the half-life is not dose dependent.
Metabolism
Iopamidol does not undergo significant metabolism, deiodination, or biotransformation.
Excretion
Iopamidol is excreted primarily through the kidneys. In patients with normal renal function, the cumulative urinary excretion for iopamidol, expressed as a percentage of administered intravenous dose, is approximately 35% to 40% at 60 minutes, 80% to 90% at 8 hours, and 90% or greater in the 72- to 96-hour period after administration. In patients with normal renal function, approximately 1% or less of the administered dose appears in cumulative 72- to 96-hour fecal samples.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed with iopamidol to evaluate carcinogenic potential. No evidence of genetic toxicity was obtained in in vitro tests. In animal reproduction studies performed on rats, intravenously administered iopamidol did not induce adverse effects on fertility or general reproductive performance.
HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Iopamidol Injection, USP is a clear, colorless to pale yellow solution available in the following presentations:
Concentration (mg Iodine/mL) | Package Size | Package Type | Sale Unit | NDC |
200 | 50 mL | Single-dose vial | Carton of 1 | 70436-211-80 |
250 | 50 mL | Single-dose vial | Carton of 10 | 70436-125-82 |
300 | 30 mL | Single-dose vial | Carton of 10 | 70436-219-52 |
300 | 50 mL | Single-dose vial | Carton of 10 | 70436-219-62 |
300 | 100 mL | Single-dose vial | Carton of 10 | 70436-219-82 |
370 | 50 mL | Single-dose vial | Carton of 10 | 70436-127-52 |
370 | 75 mL | Single-dose vial | Carton of 10 | 70436-127-62 |
370 | 100 mL | Single-dose vial | Carton of 10 | 70436-127-82 |
Storage and Handling
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Protect from light.
Mechanism of Action
Intravascular injection of iopamidol opacifies those vessels where the contrast agent is present, permitting radiographic visualization through attenuation of photons.
In imaging of the body, iodinated contrast agents diffuse from the vessels into the extravascular space. In normal brain with an intact blood-brain barrier, contrast does not diffuse into the extravascular space. In patients with a disrupted blood-brain barrier, contrast agent accumulates in the extravascular space in the region of disruption.