Get your patient on Kesimpta (Ofatumumab)
Kesimpta prior authorization resources
Most recent state uniform prior authorization forms
Brand Resources
Kesimpta patient education
Patient toolkit
Dosage & administration
2 DOSAGE AND ADMINISTRATION
2.1 Assessments Prior to First Dose of KESIMPTA
Hepatitis B Virus Screening
Prior to initiating KESIMPTA, perform Hepatitis B virus (HBV) screening. KESIMPTA is contraindicated in patients with active HBV confirmed by positive results for Hepatitis B surface antigen [HBsAg] and anti-HBV tests. For patients who are negative for HBsAg and positive for Hepatitis B core antibody [HBcAb+] or are carriers of HBV [HBsAg+], consult liver disease experts before starting and during treatment with KESIMPTA [see Warnings and Precautions (5.1)] .
Serum Immunoglobulins
Prior to initiating KESIMPTA, perform testing for quantitative serum immunoglobulins [see Warnings and Precautions (5.3)] . For patients with low serum immunoglobulins, consult immunology experts before initiating treatment with KESIMPTA.
Vaccinations
Because vaccination with live-attenuated or live vaccines is not recommended during treatment and after discontinuation until B-cell repletion, administer all immunizations according to immunization guidelines at least 4 weeks prior to initiation of KESIMPTA for live or live-attenuated vaccines, and whenever possible, at least 2 weeks prior to initiation of KESIMPTA for inactivated vaccines [see Warnings and Precautions (5.1)] .
Liver Function Tests
Prior to initiating KESIMPTA, obtain serum aminotransferases (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]), alkaline phosphatase, and bilirubin levels [see Warnings and Precautions (5.4)] .
2.2 Recommended Dosage
The recommended dosage of KESIMPTA is:
- initial dosing of 20 mg by subcutaneous injection at Weeks 0, 1, and 2, followed by
- subsequent dosing of 20 mg by subcutaneous injection once monthly starting at Week 4.
Missed Doses
If an injection of KESIMPTA is missed, it should be administered as soon as possible without waiting until the next scheduled dose. Subsequent doses should be administered at the recommended intervals.
2.3 Administration Instructions
Administer by subcutaneous injection only.
KESIMPTA is intended for patient self-administration by subcutaneous injection.
Administer KESIMPTA in the abdomen, thigh, or outer upper arm subcutaneously. Do not give injection into moles, scars, stretch marks, or areas where the skin is tender, bruised, red, scaly, or hard.
The first injection of KESIMPTA should be performed under the guidance of a healthcare professional [see Warnings and Precautions (5.2)] .
KESIMPTA Sensoready ® pens and syringes are for one-time use only and should be discarded after use. See Instructions for Use for complete administration instructions.
2.4 Preparation of KESIMPTA
The KESIMPTA “Instructions for Use” for each presentation contains more detailed instructions on the preparation of KESIMPTA.
Before administration, remove KESIMPTA Sensoready Pen or KESIMPTA prefilled syringe from the refrigerator and allow KESIMPTA to reach room temperature for about 15 to 30 minutes. DO NOT remove the needle cover while allowing the prefilled syringe to reach room temperature.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if the liquid contains visible particles or is cloudy.

By using PrescriberAI, you agree to the AI Terms of Use.
Kesimpta prescribing information
1 INDICATIONS AND USAGE
KESIMPTA is indicated for the treatment of relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults.
2 DOSAGE AND ADMINISTRATION
2.1 Assessments Prior to First Dose of KESIMPTA
Hepatitis B Virus Screening
Prior to initiating KESIMPTA, perform Hepatitis B virus (HBV) screening. KESIMPTA is contraindicated in patients with active HBV confirmed by positive results for Hepatitis B surface antigen [HBsAg] and anti-HBV tests. For patients who are negative for HBsAg and positive for Hepatitis B core antibody [HBcAb+] or are carriers of HBV [HBsAg+], consult liver disease experts before starting and during treatment with KESIMPTA [see Warnings and Precautions (5.1)] .
Serum Immunoglobulins
Prior to initiating KESIMPTA, perform testing for quantitative serum immunoglobulins [see Warnings and Precautions (5.3)] . For patients with low serum immunoglobulins, consult immunology experts before initiating treatment with KESIMPTA.
Vaccinations
Because vaccination with live-attenuated or live vaccines is not recommended during treatment and after discontinuation until B-cell repletion, administer all immunizations according to immunization guidelines at least 4 weeks prior to initiation of KESIMPTA for live or live-attenuated vaccines, and whenever possible, at least 2 weeks prior to initiation of KESIMPTA for inactivated vaccines [see Warnings and Precautions (5.1)] .
Liver Function Tests
Prior to initiating KESIMPTA, obtain serum aminotransferases (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]), alkaline phosphatase, and bilirubin levels [see Warnings and Precautions (5.4)] .
2.2 Recommended Dosage
The recommended dosage of KESIMPTA is:
- initial dosing of 20 mg by subcutaneous injection at Weeks 0, 1, and 2, followed by
- subsequent dosing of 20 mg by subcutaneous injection once monthly starting at Week 4.
Missed Doses
If an injection of KESIMPTA is missed, it should be administered as soon as possible without waiting until the next scheduled dose. Subsequent doses should be administered at the recommended intervals.
2.3 Administration Instructions
Administer by subcutaneous injection only.
KESIMPTA is intended for patient self-administration by subcutaneous injection.
Administer KESIMPTA in the abdomen, thigh, or outer upper arm subcutaneously. Do not give injection into moles, scars, stretch marks, or areas where the skin is tender, bruised, red, scaly, or hard.
The first injection of KESIMPTA should be performed under the guidance of a healthcare professional [see Warnings and Precautions (5.2)] .
KESIMPTA Sensoready ® pens and syringes are for one-time use only and should be discarded after use. See Instructions for Use for complete administration instructions.
2.4 Preparation of KESIMPTA
The KESIMPTA “Instructions for Use” for each presentation contains more detailed instructions on the preparation of KESIMPTA.
Before administration, remove KESIMPTA Sensoready Pen or KESIMPTA prefilled syringe from the refrigerator and allow KESIMPTA to reach room temperature for about 15 to 30 minutes. DO NOT remove the needle cover while allowing the prefilled syringe to reach room temperature.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use if the liquid contains visible particles or is cloudy.
3 DOSAGE FORMS AND STRENGTHS
KESIMPTA is a clear to slightly opalescent, and colorless to slightly brownish-yellow solution available as follows:
- Injection: 20 mg/0.4 mL in a single-dose prefilled Sensoready Pen
- Injection: 20 mg/0.4 mL in a single-dose prefilled syringe
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to KESIMPTA during pregnancy. Healthcare providers are encouraged to enroll pregnant patients, or pregnant women may register themselves in the MotherToBaby Pregnancy Study in Multiple Sclerosis by calling 1-877-311-8972, by sending an email to MotherToBaby@health.ucsd.edu, or by going to www.mothertobaby.org/join-study.
Risk Summary
There are no adequate data on the developmental risk associated with the use of KESIMPTA in pregnant women. Ofatumumab may cross the placenta and cause fetal B-cell depletion based on findings from animal studies ( see Data ).
Transient peripheral B-cell depletion and lymphocytopenia have been reported in infants born to mothers exposed to other anti-CD20 antibodies during pregnancy. B-cell levels in infants following maternal exposure to KESIMPTA have not been studied in clinical trials. The potential duration of B-cell depletion in infants exposed to ofatumumab in utero , and the impact of B-cell depletion on the safety and effectiveness of vaccines, are unknown. Avoid administering live vaccines to neonates and infants exposed to KESIMPTA in utero until B-cell recovery occurs [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.2)] .
Following administration of ofatumumab to pregnant monkeys, increased mortality, depletion of B-cell populations, and impaired immune function were observed in the offspring, in the absence of maternal toxicity, at plasma levels substantially higher than that in humans ( see Data ).
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Data
Animal Data
Intravenous administration of ofatumumab (weekly doses of 0, 20, or 100 mg/kg) to pregnant monkeys during the period of organogenesis (gestations days 20 to 50) resulted in no adverse effects on embryofetal development; however, B-cell depletion was observed in fetuses at both doses when assessed on gestation day 100. Plasma exposure (C ave ) at the no-effect dose (100 mg/kg) for adverse effects on embryofetal development was greater than 5000 times that in humans at the recommended human maintenance dose of 20 mg. A no-effect dose for effects on B-cells was not identified; plasma exposure (C ave ) at the low-effect dose (20 mg/kg) was approximately 780 times that in humans at the recommended human maintenance dose (RHMD) of 20 mg/month.
Intravenous administration of ofatumumab (5 weekly doses of 0, 10, and 100 mg/kg, followed by biweekly doses of 0, 3, and 20 mg/kg) to pregnant monkeys throughout pregnancy resulted in no adverse effects on the development of the offspring. However, postnatal death, B-cell depletion, and impaired immune function were observed in the offspring at the high dose. The deaths at the high dose were considered secondary to B-cell depletion. Plasma exposure (C ave ) in dams at the no-effect dose (100/20 mg/kg) for adverse developmental effects was approximately 500 times that in humans at RHMD. A no-effect level for mortality and immune effects in offspring was not established because of the limited number of evaluable offspring at the low dose.
8.2 Lactation
Risk Summary
There are no data on the presence of ofatumumab in human milk, the effects on the breastfed infant, or the effects of the drug on milk production. Human IgG is excreted in human milk, and the potential for absorption of ofatumumab to lead to B-cell depletion in the infant is unknown. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for KESIMPTA and any potential adverse effects on the breastfed infant from KESIMPTA or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Contraception
Females of childbearing potential should use effective contraception while receiving KESIMPTA and for 6 months after the last treatment of KESIMPTA [see Warnings and Precautions (5.5) and Clinical Pharmacology (12.3)] .
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
8.5 Geriatric Use
Clinical studies of KESIMPTA did not include sufficient numbers of geriatric patients to determine whether they respond differently from younger subjects.
4 CONTRAINDICATIONS
KESIMPTA is contraindicated in patients with:
- Active HBV infection [see Warnings and Precautions (5.1)] .
- History of hypersensitivity to ofatumumab or life-threatening injection-related reaction to KESIMPTA. Hypersensitivity reactions have included anaphylaxis and angioedema [see Warnings and Precautions (5.2)] .
5 WARNINGS AND PRECAUTIONS
- Infections: Serious, including life-threatening and fatal infections, have occurred in patients treated with anti-CD20 therapies. Delay KESIMPTA administration in patients with an active infection until the infection is resolved. Vaccination with live-attenuated or live vaccines is not recommended during treatment with KESIMPTA and after discontinuation, until B-cell repletion. (5.1 )
- Injection-Related Reactions and Hypersensitivity Reactions: Management for injection-related reactions and hypersensitivity reactions depends on the type and severity of the reaction. (4 , 5.2 )
- Reduction in Immunoglobulins: Monitor the level of immunoglobulins at the beginning, during, and after discontinuation of treatment with KESIMPTA until B-cell repletion. Consider discontinuing KESIMPTA if a patient develops a serious opportunistic infection or recurrent infections if immunoglobulin levels indicate immune compromise. (5.3 )
- Liver Injury: Clinically significant liver injury has occurred. Obtain serum aminotransferases, alkaline phosphatase, and bilirubin levels before initiating KESIMPTA, and during treatment as clinically indicated. Discontinue KESIMPTA in patients with evidence of liver injury in the absence of an alternative etiology. (5.4 )
- Fetal Risk: May cause fetal harm based on animal data. Advise females of reproductive potential of the potential risk to a fetus and to use an effective method of contraception during treatment and for 6 months after stopping KESIMPTA. (5.5 , 8.1 )
5.1 Infections
Serious, including life-threatening or fatal, bacterial, fungal, and new or reactivated viral infections have been observed during and following completion of treatment with anti-CD20 B-cell depleting therapies.
In KESIMPTA Study 1 and Study 2 [see Clinical Studies (14)] , the overall rate of infections and serious infections in patients treated with KESIMPTA was similar to patients who were treated with teriflunomide (51.6% vs 52.7%, and 2.5% vs 1.8%, respectively). The most common infections reported by KESIMPTA-treated patients in the randomized clinical relapsing MS (RMS) trials included upper respiratory tract infection (39%) and urinary tract infection (10%). Delay KESIMPTA administration in patients with an active infection until the infection is resolved.
Possible Increased Risk of Immunosuppressant Effects with Other Immunosuppressants
When initiating KESIMPTA after an immunosuppressive therapy or initiating an immunosuppressive therapy after KESIMPTA, consider the potential for increased immunosuppressive effects [see Drug Interactions (7.1) and Clinical Pharmacology (12.2)] . KESIMPTA has not been studied in combination with other MS therapies.
Hepatitis B Virus
Reactivation
There were no reports of HBV reactivation in patients with MS treated with KESIMPTA. However, HBV reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death, has occurred in patients being treated with ofatumumab for chronic lymphocytic leukemia (CLL) (at higher intravenous doses than the recommended dose in MS but for a shorter duration of treatment) and in patients treated with other anti-CD20 antibodies.
Infection
KESIMPTA is contraindicated in patients with active hepatitis B disease. Fatal infections caused by HBV in patients who have not been previously infected have occurred in patients being treated with ofatumumab for CLL (at higher intravenous doses than the recommended dose in MS but for a shorter duration of treatment). HBV screening should be performed in all patients before initiation of treatment with KESIMPTA. At a minimum, screening should include Hepatitis B surface antigen (HBsAg) and Hepatitis B Core Antibody (HBcAb) testing. These can be complemented with other appropriate markers as per local guidelines. For patients who are negative for HBsAg and positive for HB core antibody [HBcAb+] or are carriers of HBV [HBsAg+], consult liver disease experts before starting and during treatment with KESIMPTA. These patients should be monitored and managed following local medical standards to prevent HBV infection or reactivation.
Progressive Multifocal Leukoencephalopathy
Progressive multifocal leukoencephalopathy (PML) is an opportunistic viral infection of the brain caused by the JC virus (JCV) that typically occurs in patients who are immunocompromised, and that usually leads to death or severe disability.
Although no cases of PML have been reported for KESIMPTA in the RMS clinical studies, PML resulting in death has occurred in patients being treated with ofatumumab for CLL (at substantially higher intravenous doses than the recommended dose in MS but for a shorter duration of treatment). In addition, JCV infection resulting in PML has also been observed in patients treated with other anti-CD20 antibodies and other MS therapies. At the first sign or symptom suggestive of PML, withhold KESIMPTA and perform an appropriate diagnostic evaluation. Magnetic resonance imaging (MRI) findings may be apparent before clinical signs or symptoms. Typical symptoms associated with PML are diverse, progress over days to weeks, and include progressive weakness on one side of the body or clumsiness of limbs, disturbance of vision, and changes in thinking, memory, and orientation leading to confusion and personality changes.
If PML is confirmed, treatment with KESIMPTA should be discontinued.
Vaccinations
Administer all immunizations according to immunization guidelines at least 4 weeks prior to initiation of KESIMPTA for live or live-attenuated vaccines, and whenever possible, at least 2 weeks prior to initiation of KESIMPTA for inactivated vaccines.
KESIMPTA may interfere with the effectiveness of inactivated vaccines.
The safety of immunization with live or live-attenuated vaccines following KESIMPTA therapy has not been studied. Vaccination with live or live-attenuated vaccines is not recommended during treatment and after discontinuation until B-cell repletion [see Clinical Pharmacology (12.2)] .
Vaccination of Infants Born to Mothers Treated with KESIMPTA During Pregnancy
In infants of mothers treated with KESIMPTA during pregnancy, do not administer live or live-attenuated vaccines before confirming the recovery of B-cell counts. Depletion of B-cells in these infants may increase the risks from live or live-attenuated vaccines.
Inactivated vaccines may be administered, as indicated, prior to recovery from B-cell depletion, but an assessment of vaccine immune responses, including consultation with a qualified specialist, should be considered to determine whether a protective immune response was mounted.
5.2 Injection-Related Reactions and Hypersensitivity Reactions
KESIMPTA can result in systemic injection-related reactions and hypersensitivity reactions, which may be serious or life-threatening.
In Study 1 and Study 2, systemic and local injection reactions were reported in 21% and 11% of patients treated with KESIMPTA, compared to 15% and 6% of patients treated with teriflunomide who received matching placebo injections, respectively [see Adverse Reactions (6.1) and Clinical Studies (14)] .
Injection-related reactions with systemic symptoms observed in clinical studies occurred most commonly within 24 hours of the first injection, but were also observed with later injections. Symptoms observed included fever, headache, myalgia, chills, and fatigue, and were predominantly (99.8%) mild to moderate in severity. There were no life-threatening injection reactions in the RMS clinical studies.
In the post-marketing setting, additional systemic injection-related reactions and hypersensitivity reactions have been reported, including anaphylaxis, angioedema, pruritus, rash, urticaria, erythema, bronchospasm, throat irritation, oropharyngeal pain, dyspnea, pharyngeal or laryngeal edema, flushing, hypotension, dizziness, nausea, and tachycardia. Most cases were non-serious and occurred with the first injection. Most serious cases resulted in permanent discontinuation of KESIMPTA.
Symptoms of systemic injection-related reactions may be clinically indistinguishable from acute hypersensitivity reactions. A hypersensitivity reaction may occur with any injection. New or more severe symptoms compared to those experienced with previous injections should prompt consideration of a potential hypersensitivity reaction.
Only limited benefit of premedication with corticosteroids, antihistamines, or acetaminophen was observed in RMS clinical studies. The first injection of KESIMPTA should be performed under the guidance of an appropriately trained healthcare professional. If systemic injection-related reactions occur, initiate appropriate therapy. Patients who experience symptoms of systemic injection-related reactions or hypersensitivity reactions with KESIMPTA should be instructed to seek immediate medical attention.
If a hypersensitivity reaction or life-threatening systemic injection-related reaction occurs, immediately and permanently discontinue KESIMPTA [see Contraindications (4)] . If restarting KESIMPTA after a severe (but not life-threatening) systemic injection-related reaction or other event after which rechallenge is considered appropriate, administer the next KESIMPTA injection under clinical observation. If a mild to moderate injection-related reaction occurs, consider rechallenge under clinical observation.
Local injection-site reaction symptoms observed in clinical studies included erythema, swelling, itching, and pain. If local injection-related reactions occur, symptomatic treatment is recommended.
5.3 Reduction in Immunoglobulins
As expected with any B-cell depleting therapy, decreased immunoglobulin levels were observed. Decrease in immunoglobulin M (IgM) was reported in 7.7% of patients treated with KESIMPTA compared to 3.1% of patients treated with teriflunomide in RMS clinical trials [see Adverse Reactions (6.1)] . Treatment was discontinued because of decreased immunoglobulins in 3.4% of patients treated with KESIMPTA and in 0.8% of patients treated with teriflunomide. No decline in immunoglobulin G (IgG) was observed at the end of the study. Monitor the levels of quantitative serum immunoglobulins during treatment, especially in patients with opportunistic or recurrent infections, and after discontinuation of therapy until B-cell repletion. Consider discontinuing KESIMPTA therapy if a patient with low immunoglobulins develops a serious opportunistic infection or recurrent infections, or if prolonged hypogammaglobulinemia requires treatment with intravenous immunoglobulins.
5.4 Liver Injury
Clinically significant liver injury, without findings of viral hepatitis, has been reported in the postmarketing setting in patients treated with anti-CD20 B-cell depleting therapies approved for the treatment of MS, including KESIMPTA. Signs of liver injury, including markedly elevated serum hepatic enzymes with elevated total bilirubin, have occurred weeks to months after administration.
Patients treated with KESIMPTA found to have an alanine aminotransferase (ALT) or aspartate aminotransferase (AST) greater than 3x the upper limit of normal (ULN) with serum total bilirubin greater than 2x ULN, are potentially at risk for severe drug-induced liver injury.
Obtain liver function tests prior to initiating treatment with KESIMPTA [see Dosage and Administration (2.1)] , and monitor for signs and symptoms of any hepatic injury during treatment. Measure serum aminotransferases, alkaline phosphatase, and bilirubin levels promptly in patients who report symptoms that may indicate liver injury, including new or worsening fatigue, anorexia, nausea, vomiting, right upper abdominal discomfort, dark urine, or jaundice. If liver injury is present and an alternative etiology is not identified, discontinue KESIMPTA.
5.5 Fetal Risk
Based on animal data, KESIMPTA can cause fetal harm due to B-cell lymphopenia and reduce antibody response in offspring exposed to KESIMPTA in utero . Transient peripheral B-cell depletion and lymphocytopenia have been reported in infants born to mothers exposed to other anti-CD20 B-cell depleting antibodies during pregnancy. Advise females of reproductive potential to use effective contraception while receiving KESIMPTA and for at least 6 months after the last dose [see Use in Specific Populations (8.1)] .
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail elsewhere in the labeling:
- Infections [see Warnings and Precautions (5.1)]
- Injection-Related Reactions and Hypersensitivity Reactions [see Warnings and Precautions (5.2)]
- Reduction in Immunoglobulins [see Warnings and Precautions (5.3)]
- Liver Injury [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Approximately 1500 patients with RMS received KESIMPTA in clinical studies. In Study 1 and Study 2, 1882 patients with RMS were randomized, 946 of whom were treated with KESIMPTA for a median duration of 85 weeks; 33% of patients receiving KESIMPTA were treated for up to 120 weeks [see Clinical Studies (14.1)] . The most common adverse reactions occurring in greater than 10% of patients treated with KESIMPTA and more frequently than in patients treated with teriflunomide were upper respiratory tract infections, injection-related reactions (systemic), headache, and injection-site reactions (local). The most common cause of discontinuation in patients treated with KESIMPTA was low immunoglobulin M (3.3%), defined in trial protocols as IgM at 10% below the lower limit of normal (LLN).
Table 1 summarizes the adverse drug reactions that occurred in Study 1 and Study 2.
| a Includes the following: nasopharyngitis, upper respiratory tract infection, influenza, sinusitis, pharyngitis, rhinitis, viral upper respiratory infection, tonsillitis, acute sinusitis, pharyngotonsillitis, laryngitis, pharyngitis streptococcal, viral rhinitis, sinusitis bacterial, tonsillitis bacterial, viral pharyngitis, viral tonsillitis, chronic sinusitis, nasal herpes, tracheitis. | ||
| Adverse reactions | KESIMPTA 20 mg N = 946 % | Teriflunomide 14 mg N = 936 % |
| Upper respiratory tract infections a | 39 | 38 |
| Injection-related reactions (systemic) | 21 | 15 |
| Headache | 13 | 12 |
| Injection-site reactions (local) | 11 | 6 |
| Urinary tract infection | 10 | 8 |
| Back pain | 8 | 6 |
| Blood immunoglobulin M decreased | 6 | 2 |
Injection-Related Reactions and Injection-Site Reactions
The incidence of injection-related reactions (systemic) was highest with the first injection (14.4%), decreasing with subsequent injections (4.4% with second, less than 3% with third injection). Injection-related reactions were mostly (99.8%) mild to moderate in severity. Two (0.2%) patients treated with KESIMPTA reported serious injection-related reactions. There were no life-threatening injection-related reactions. Most frequently reported symptoms (2% or greater) included fever, headache, myalgia, chills, and fatigue.
In addition to systemic injection-related reactions, local reactions at the administration site were very common. Local injection-site reactions were all mild to moderate in severity. The most frequently reported symptoms (2% or greater) included erythema, pain, itching, and swelling [see Warnings and Precautions (5.2)] .
Laboratory Abnormalities
Immunoglobulins
In Study 1 and Study 2, a decrease in the mean level of IgM was observed in KESIMPTA-treated patients but was not associated with an increased risk of infections [see Warnings and Precautions (5.3)] . In 14.3% of patients in Study 1 and Study 2, treatment with KESIMPTA resulted in a decrease in a serum IgM that reached a value below 0.34 g/L. KESIMPTA was associated with a decrease of 4.3% in mean IgG levels after 48 weeks of treatment and an increase of 2.2% after 96 weeks.
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medication, and the underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other ofatumumab products may be misleading.
Treatment-induced anti-drug antibodies (ADAs) were detected in 2 of 914 (0.2%) KESIMPTA-treated patients; no patients with treatment-enhancing or neutralizing ADAs were identified. There was no impact of positive ADA titers on pharmacokinetics, safety profile or B-cell kinetics in any patient; however, these data are not adequate to assess the impact of ADAs on the safety and efficacy of KESIMPTA.
6.3 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of KESIMPTA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System: Hypersensitivity reactions [see Warnings and Precautions (5.2)]
Hepatobiliary Disorders: Liver injury [see Warnings and Precautions (5.4)]
7 DRUG INTERACTIONS
7.1 Immunosuppressive or Immune-Modulating Therapies
Concomitant usage of KESIMPTA with immunosuppressant drugs, including systemic corticosteroids, may increase the risk of infection. Consider the risk of additive immune system effects when coadministering immunosuppressive therapies with KESIMPTA.
When switching from therapies with immune effects, the duration and mechanism of action of these therapies should be taken into account because of potential additive immunosuppressive effects when initiating KESIMPTA.
11 DESCRIPTION
Ofatumumab is a recombinant human monoclonal immunoglobulin G1 (IgG1) antibody that binds to human CD20 expressed on B-cells. Ofatumumab is produced in a murine NS0 cell line and consists of two IgG1 heavy chains and two kappa light chains with a molecular weight of approximately 146 kDa.
KESIMPTA (ofatumumab) injection is a sterile, preservative-free solution for subcutaneous use.
Each 20 mg/0.4 mL KESIMPTA Sensoready Pen or prefilled syringe delivers 0.4 mL of solution. Each 0.4 mL contains 20 mg of ofatumumab, and arginine (4 mg), disodium edetate (0.007 mg), polysorbate 80 (0.08 mg), sodium acetate trihydrate (2.722 mg), sodium chloride (1.192 mg), and Water for Injection, USP with a pH of 5.5. Hydrochloric acid may have been added to adjust pH.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The precise mechanism by which ofatumumab exerts its therapeutic effects in multiple sclerosis is unknown, but is presumed to involve binding to CD20, a cell surface antigen present on pre-B and mature B lymphocytes. Following cell surface binding to B lymphocytes, ofatumumab results in antibody-dependent cellular cytolysis and complement-mediated lysis.
12.2 Pharmacodynamics
B-cell Depletion
For B-cell counts, assays for CD19+ B-cells are used because the presence of ofatumumab interferes with the CD20 assay. In Study 1 and Study 2, KESIMPTA administered as recommended, resulted in a reduction of CD19+ B-cells to below the LLN in 77.0% and 78.8% of patients, respectively, one week after treatment initiation, and in 95.0% and 95.8% of patients, respectively, two weeks after treatment initiation [see Dosage and Administration (2.2) and Clinical Studies (14)] . In Study 1 and Study 2, at Week 12, 99.3% to 99.5% of patients had CD19+ B-cell counts below LLN. The CD19+ B-cell counts remained below LLN for approximately 97% of patients in Study 1 and 92% of patients in Study 2 from 12 weeks through 120 weeks while on KESIMPTA treatment.
In a study of bioequivalence using the same dosing regimen as in Study 1 and Study 2, before initiation of the maintenance phase, total CD19+ B-cell levels below the defined threshold of 10 cells/µL were achieved in 94% of patients starting at Week 4 and 98% of patients at Week 12.
B-cell Repletion
Data from Studies 1 and 2 indicate a median time to B-cell recovery to either LLN or baseline value of 24.6 weeks post-treatment discontinuation. Pharmacokinetics and pharmacodynamics modeling and simulation for B-cell repletion corroborate these data, predicting median time to B-cell recovery to LLN of 23 weeks post-treatment discontinuation.
12.3 Pharmacokinetics
Absorption
A subcutaneous dose of 20 mg every 4 weeks leads to a mean AUC tau of 483 mcg h/mL and a mean C max of 1.43 mcg/mL at steady state.
After subcutaneous administration, ofatumumab is believed to be predominantly absorbed via the lymphatic system similarly to other therapeutic monoclonal antibodies.
Distribution
The volume of distribution at steady-state was estimated to be 5.42 L following subcutaneous administration of repeated KESIMPTA 20 mg dose.
Elimination
Ofatumumab is eliminated by both linear catabolic pathways and a non-linear B-cell mediated pathway, resulting in non-constant elimination half-life. Initially, a higher baseline B-cell count results in greater B-cell-mediated elimination and shorter ofatumumab half-life. As the B-cells are depleted, the catabolic pathway predominates, resulting in a relatively longer ofatumumab half-life compared to earlier in therapy when both elimination pathways were available. Following B-cell depletion, clearance was estimated to be 0.34 L/day following repeated subcutaneous administration of KESIMPTA 20 mg injections. The half-life at steady state was estimated to be approximately 16 days following subcutaneous administration of repeated KESIMPTA 20 mg dosage.
Metabolism
Ofatumumab is a protein for which the expected metabolic pathway is degradation to small peptides and amino acids by ubiquitous proteolytic enzymes.
Excretion
Ofatumumab, a monoclonal antibody, is not likely to undergo renal excretion.
Specific Populations
The following population characteristics do not have a clinically meaningful effect on the pharmacokinetics of ofatumumab: body weight, sex, age, race, or baseline B-cell count.
Patients with Renal/Hepatic Impairment
Pharmacokinetics of ofatumumab in patients with renal or hepatic impairment have not been studied.
Drug Interaction Studies
Ofatumumab does not share a common clearance pathway with chemical drugs that are metabolized by the cytochrome P450 system or other drug-metabolizing enzymes. Additionally, there is no evidence that CD20 monoclonal antibodies are involved in the regulation of the expression of drug-metabolizing enzymes. Interactions between KESIMPTA and other medicinal products have not been investigated in formal studies.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No carcinogenicity studies have been conducted to assess the carcinogenic potential of ofatumumab.
Mutagenesis
No studies have been conducted to assess the mutagenic potential of ofatumumab. As an antibody, ofatumumab is not expected to interact directly with DNA.
Impairment of Fertility
No effects on reproductive parameters, including hormones, menstrual cycle, sperm analysis, or histopathological evaluation of reproductive organs, were observed in male or female monkeys administered ofatumumab by intravenous injection (5 weekly doses of 0, 10, and 100 mg/kg, followed by biweekly doses of 0, 3, and 20 mg/kg). Plasma exposures (C ave ) at the high dose tested in monkeys are greater than 500 times that in humans at the recommended human maintenance dose of 20 mg/month.
14 CLINICAL STUDIES
The efficacy of KESIMPTA was demonstrated in two randomized, double-blind, double-dummy, active comparator-controlled clinical trials of identical design, in patients with relapsing forms of MS [Study 1 (NCT02792218) and Study 2 (NCT02792231)]. Both studies enrolled patients with at least one relapse in the previous year, 2 relapses in the previous 2 years, or the presence of a T1 gadolinium-enhancing (GdE) lesion in the previous year. Patients were also required to have an Expanded Disability Status Scale (EDSS) score from 0 to 5.5.
Patients were randomized to receive either KESIMPTA, 20 mg subcutaneously on Days 1, 7, and 14, followed by 20 mg every 4 weeks thereafter starting at Week 4 with a daily oral placebo, or the active comparator, teriflunomide, at a dose of 14 mg orally once daily with a placebo administered subcutaneously on Days 1, 7, 14, and every 4 weeks thereafter. The treatment duration for an individual patient was variable based on when the end-of-study criteria were met. The maximal duration of treatment for an individual patient was 120 weeks. Neurologic evaluations were performed at baseline, every 3 months during blinded treatment, and at the time of a suspected relapse. Brain MRI scans were performed at baseline, 1 year, and 2 years.
The primary endpoint of both trials was the annualized relapse rate (ARR) over the treatment period. Additional outcome measures included: 1) the time to 3-month confirmed disability progression for the pooled populations, 2) the number of T1 GdE lesions per scan at Weeks 48 and 96, and 3) the annualized rate of new or enlarging T2 MRI lesions. Disability progression was defined as an increase in EDSS of at least 1.5, 1, or 0.5 points in patients with a baseline EDSS of 0, 1 to 5, or 5.5 or greater, respectively.
In Study 1, a total of 927 patients were randomized to receive KESIMPTA (n = 465) or teriflunomide (n = 462). Of those randomized to KESIMPTA, 90% completed the study; of those randomized to teriflunomide, 81% completed the study. Demographics and disease characteristics were balanced across treatment arms. The mean age was 38 years, 89% were White, and 69% were female. The mean time since MS diagnosis was 5.7 years and the median EDSS score at baseline was 3.0; 60% had been treated with a non-steroid therapy for MS. At baseline, the mean number of relapses in the previous year was 1 and the mean number of T1 GdE lesions on MRI scan was 1.5.
In Study 2, a total of 955 patients were randomized to receive KESIMPTA (n = 481) or teriflunomide (n = 474). Of those randomized to KESIMPTA, 83% completed the study; of those randomized to teriflunomide, 82% completed the study. Demographics and disease characteristics were balanced across treatment arms. The mean age was 38 years, 87% were White, and 67% were female. The mean time since MS diagnosis was 5.5 years and the median EDSS score at baseline was 2.5; 61% had been treated with a non-steroid therapy for MS. At baseline, the mean number of relapses in the previous year was 1.3, and the mean number of T1 GdE lesions on MRI scan was 1.6.
In both studies, KESIMPTA significantly lowered the ARR compared to teriflunomide.
KESIMPTA significantly reduced the risk of 3-month confirmed disability progression compared to teriflunomide.
KESIMPTA significantly reduced the number of T1 GdE lesions and the rate of new or enlarging T2 lesions in both studies.
Key results for Study 1 and Study 2 are presented in Table 2 and Figure 1.
| a Disability progression was defined as an increase in EDSS of at least 1.5, 1, or 0.5 points in patients with a baseline EDSS of 0, 1 to 5, or 5.5 or greater, respectively. b Prospective pooled analysis of Studies 1 and 2. Proportion of patients with 3-month confirmed disability progression refers to Kaplan-Meier estimates at Month 24. | ||||
| Study 1 | Study 2 | |||
| Endpoints | KESIMPTA 20 mg (n = 465) | Teriflunomide 14 mg (n = 462) | KESIMPTA 20 mg (n = 481) | Teriflunomide 14 mg (n = 474) |
| Clinical Endpoints | ||||
| Annualized relapse rate (primary endpoint) | 0.11 | 0.22 | 0.10 | 0.25 |
| Relative reduction | 51% ( p < 0.001) | 58% ( p < 0.001) | ||
| Proportion of patients with 3-month confirmed disability progression a,b Relative risk reduction | 10.9% KESIMPTA vs 15.0% teriflunomide 34.3% ( p = 0.003) | |||
| MRI Endpoints | ||||
| Mean number of T1 Gd-enhancing lesions per MRI scan | 0.01 | 0.46 | 0.03 | 0.52 |
| Relative reduction | 98% ( p < 0.001) | 94% ( p < 0.001) | ||
| Number of new or enlarging T2 lesions per year | 0.72 | 4.00 | 0.64 | 4.16 |
| Relative reduction | 82% ( p < 0.001) | 85% ( p < 0.001) | ||
Figure 1: Time to First 3-month Confirmed Disability Progression by Treatment Full Analysis Set

A similar effect of KESIMPTA on the key efficacy results compared to teriflunomide was observed across the two studies in exploratory subgroups defined by sex, age, body weight, prior non-steroid MS therapy, and baseline disability and disease activity.
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
KESIMPTA (ofatumumab) injection is a preservative-free, clear to slightly opalescent and colorless to slightly brownish-yellow solution for subcutaneous administration, which is supplied as follows:
KESIMPTA Sensoready Pen:
Carton of one 20 mg/0.4 mL single-dose prefilled Sensoready Pen NDC 0078-1007-68
KESIMPTA Prefilled Syringe:
Carton of one 20 mg/0.4 mL single-dose prefilled syringe NDC 0078-1007-69
16.2 Storage and Handling
KESIMPTA Sensoready pens and prefilled syringes must be refrigerated at 2ºC to 8ºC (36ºF to 46ºF). Keep the product in the original carton to protect from light until the time of use. Do not freeze. To avoid foaming, do not shake.
If necessary, KESIMPTA may be stored for up to 7 days at room temperature, not to exceed 30°C (86°F). Write the date removed from the refrigerator in the space provided on the carton labeling. If stored below 30°C (86°F), unused KESIMPTA may be returned to the refrigerator and must be used within the next 7 days or discarded after 7 days.
| This Instructions for Use has been approved by the U.S. Food and Drug Administration. | Revised: 9/2022 |
| INSTRUCTIONS FOR USE KESIMPTA ® [KEY-simp-ta] (ofatumumab) injection, for subcutaneous use Sensoready ® Pen | |
This Instructions for Use contains information on how to inject KESIMPTA Sensoready Pen. Be sure that you read, understand, and follow this Instructions for Use before injecting KESIMPTA. Your healthcare provider should show you how to prepare and inject KESIMPTA the right way using the Sensoready Pen before you use it for the first time. Talk to your healthcare provider if you have any questions before you use KESIMPTA for the first time. Important Information You Need to Know Before Injecting KESIMPTA Sensoready Pen.
| |
How should I store KESIMPTA Sensoready Pen?
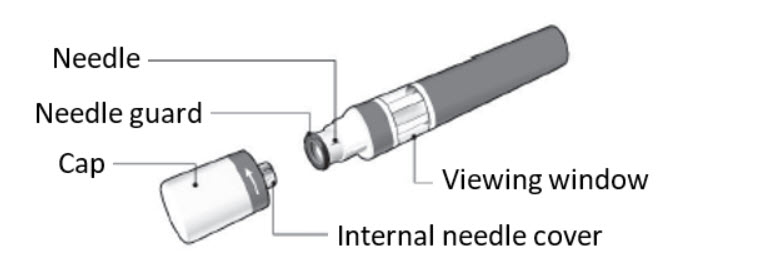 The KESIMPTA Sensoready Pen is shown with the cap removed. Do not remove the cap until you are ready to inject. The KESIMPTA Sensoready Pen is shown with the cap removed. Do not remove the cap until you are ready to inject. | |
| What you need for your injection: Included in the carton: A new KESIMPTA Sensoready Pen ( see Figure B ). | Figure B  |
Not included in the carton ( see Figure C ):
| Figure C  |
Before your injection Take the KESIMPTA Sensoready Pen out of the refrigerator 15 to 30 minutes before injecting to allow it to reach room temperature. Step 1. Important safety checks before you inject ( see Figure D ):
| Figure D 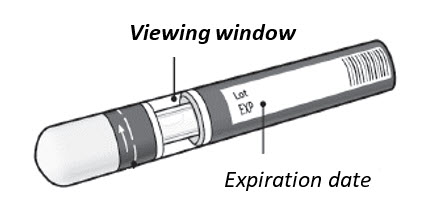 |
Step 2. Choose your injection site:
| Figure E 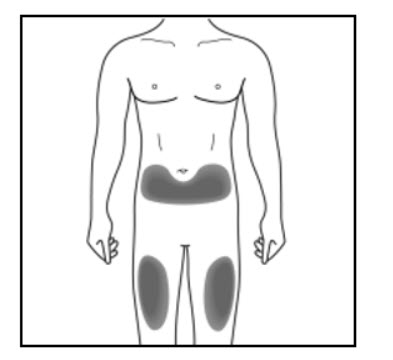 Figure F (Caregiver and healthcare provider only) Figure F (Caregiver and healthcare provider only) 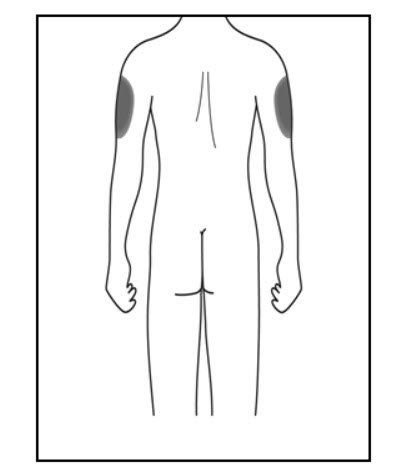 |
Step 3. Clean your injection site:
| Figure G 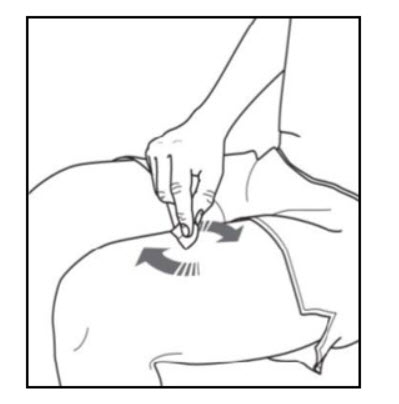 |
Your injection Step 4. Remove the cap:
| Figure H 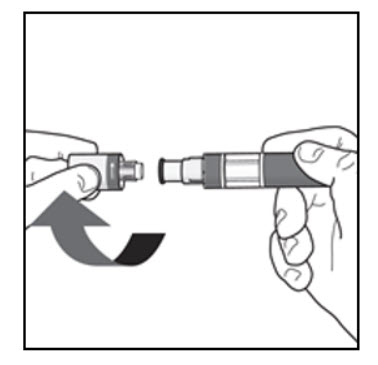 |
Step 5. Hold your KESIMPTA Sensoready Pen:
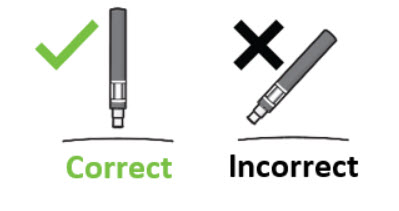 | Figure I 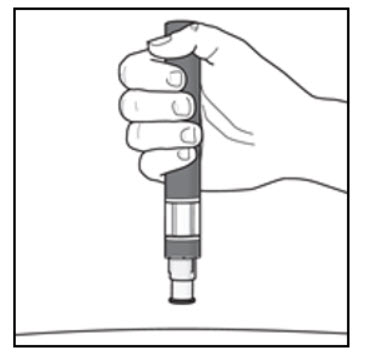 |
Important: During the injection you will hear 2 loud clicks:
| |
Step 6. Start your injection:
| Figure J 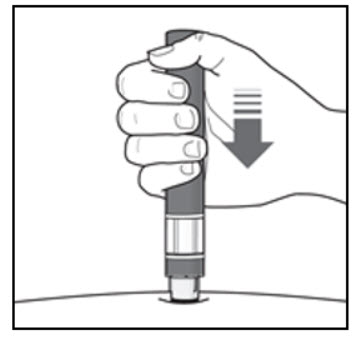 |
Step 7. Complete your injection:
| Figure K 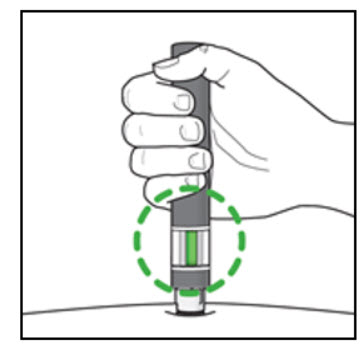 |
After your injection
| Figure L 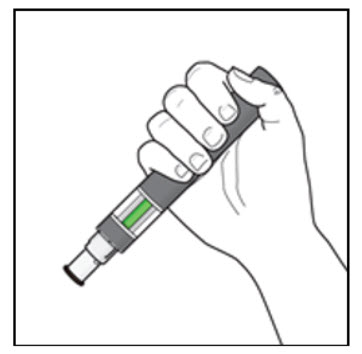 |
How should I dispose of used KESIMPTA Sensoready ® Pens? Step 8. Put your used KESIMPTA Sensoready Pen in an FDA-cleared sharps disposal container right away after use ( see Figure M ). Do not throw away (dispose of) your used KESIMPTA Sensoready Pen in your household trash. If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
| Figure M 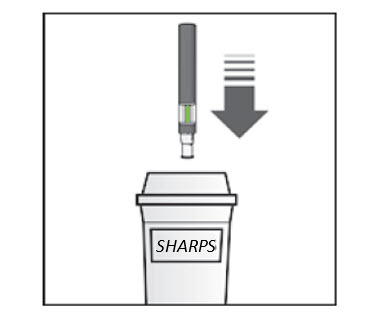 |
T2022-54
12.1 Mechanism of Action
The precise mechanism by which ofatumumab exerts its therapeutic effects in multiple sclerosis is unknown, but is presumed to involve binding to CD20, a cell surface antigen present on pre-B and mature B lymphocytes. Following cell surface binding to B lymphocytes, ofatumumab results in antibody-dependent cellular cytolysis and complement-mediated lysis.