Konvomep
(Omeprazole And Sodium Bicarbonate)Dosage & Administration
Recommended doses of KONVOMEP in the table below are based upon the omeprazole content. (
The recommended dosage regimen in adults of KONVOMEP by indication is summarized in Table 1. Recommended dosage is based upon the omeprazole content of KONVOMEP.
| Indication | Recommended Dosage | Treatment Duration |
|---|---|---|
Treatment of Benign Gastric Ulcer | 40 mg once daily | 4 to 8 weeks |
Reduction of Risk of Upper GI Bleeding in Critically Ill Patients | 40 mg initially; followed by 40 mg 6 to 8 hours later; and 40 mg once daily thereafter | 14 days |
Indication | Recommended Adult Dosage ( The recommended dosage regimen in adults of KONVOMEP by indication is summarized in Table 1. Recommended dosage is based upon the omeprazole content of KONVOMEP.
| |||||||||
Active Benign Gastric Ulcer | 40 mg once daily for 4 to 8 weeks | |||||||||
Reduction of Risk of Upper GI Bleeding in Critically Ill Patients | 40 mg initially followed by 40 mg 6 to 8 hours later and 40 mg once daily thereafter for 14 days |
By using PrescriberAI, you agree to the AI Terms of Use.
Konvomep Prescribing Information
KONVOMEP is indicated in adults for:
- short-term treatment (4 to 8 weeks) of active benign gastric ulcer.
- reduction of risk of upper gastrointestinal (GI) bleeding in critically ill adult patients.
Recommended doses of KONVOMEP in the table below are based upon the omeprazole content. (
The recommended dosage regimen in adults of KONVOMEP by indication is summarized in Table 1. Recommended dosage is based upon the omeprazole content of KONVOMEP.
| Indication | Recommended Dosage | Treatment Duration |
|---|---|---|
Treatment of Benign Gastric Ulcer | 40 mg once daily | 4 to 8 weeks |
Reduction of Risk of Upper GI Bleeding in Critically Ill Patients | 40 mg initially; followed by 40 mg 6 to 8 hours later; and 40 mg once daily thereafter | 14 days |
Indication | Recommended Adult Dosage ( The recommended dosage regimen in adults of KONVOMEP by indication is summarized in Table 1. Recommended dosage is based upon the omeprazole content of KONVOMEP.
| |||||||||
Active Benign Gastric Ulcer | 40 mg once daily for 4 to 8 weeks | |||||||||
Reduction of Risk of Upper GI Bleeding in Critically Ill Patients | 40 mg initially followed by 40 mg 6 to 8 hours later and 40 mg once daily thereafter for 14 days |
For Oral Suspension: 2 mg omeprazole and 84 mg sodium bicarbonate per mL of a pink to red hazy, strawberry-flavored liquid after reconstitution in 90 mL, 150 mL, or 300 mL bottles. Each kit contains a bottle of omeprazole as a white to off-white powder and a strawberry-flavored diluent containing sodium bicarbonate as a slightly hazy red liquid
KONVOMEP (omeprazole and sodium bicarbonate for oral suspension) is a combination of omeprazole, a PPI, and sodium bicarbonate, an antacid. Omeprazole is a substituted benzimidazole, 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1
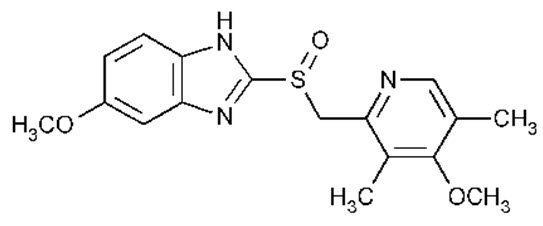
Omeprazole is a white to off-white crystalline powder which melts with decomposition at about 155°C. It is a weak base, freely soluble in ethanol and methanol, slightly soluble in acetone and isopropanol and very slightly soluble in water. The stability of omeprazole is a function of pH; it is rapidly degraded in acid media but has acceptable stability under alkaline conditions. Sodium bicarbonate raises the gastric pH and protects the omeprazole from acid degradation.
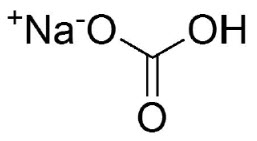
Sodium bicarbonate is a white crystalline powder. It is soluble in water and insoluble in alcohol. One gram of sodium bicarbonate dissolves in 12 mL of water at 25ºC. The pH of a freshly prepared 0.1 molar aqueous solution is 8.3 at 25ºC. Aqueous solutions slowly convert to sodium carbonate through loss of carbon dioxide.
The molecular formula is NaHCO3and the molecular weight is 84.01. The structural formula is:
KONVOMEP is supplied as a kit. Each kit is comprised of 1 bottle of pre‑weighed omeprazole powder and 1 bottle of pre‑measured strawberry‑flavored diluent to be reconstituted for oral administration. The strawberry‑flavored diluent contains sodium bicarbonate and the following inactive ingredients: benzyl alcohol, carboxymethylcellulose sodium, FD&C Red No. 40, poloxamer 188, purified water, simethicone emulsion, sodium citrate (dihydrate), sorbitol solution, strawberry flavor (natural and artificial flavors, propylene glycol, and glycerin), and sucralose. After reconstitution each mL contains 2 mg omeprazole and 84 mg sodium bicarbonate.


KONVOMEP (omeprazole and sodium bicarbonate for oral suspension) 2 mg/84 mg per mL is supplied as:
A kit containing two bottles:
- one bottle with child resistant closure of omeprazole USP, a white to off-white powder and
- one bottle of pre‑measured strawberry‑flavored slightly hazy red diluent containing sodium bicarbonate (see table below).
Prior to dispensing, reconstitute KONVOMEP for oral suspension
| Final Volume of KONVOMEP after reconstitution | Kit Contents | NDC Numbers |
|---|---|---|
90 mL | Bottle of 0.18 g omeprazole powder | 65628-270-03 |
Bottle of diluent containing sodium bicarbonate 7.56 g per 90 mL | 65628-271-03 | |
Konvomep Kit | 65628-272-03 | |
150 mL | Bottle of 0.3 g omeprazole powder | 65628-270-05 |
Bottle of diluent containing sodium bicarbonate 12.6 g per 150 mL | 65628-271-05 | |
Konvomep Kit | 65628-272-05 | |
300 mL | Bottle of 0.6 g omeprazole powder | 65628-270-10 |
Bottle of diluent containing sodium bicarbonate 25.2 g per 300 mL | 65628-271-10 | |
Konvomep Kit | 65628-272-10 |
- Store KONVOMEP kit in the refrigerator, 2°C to 8°C (36°F to 46°F).
- Store reconstituted suspension of KONVOMEP in the refrigerator, 2°C to 8°C (36°F to 46°F); discard unused reconstituted suspension after 30 days.
- Keep containers tightly closed.
- Protect containers from light.
- Protect containers from freezing.
There are no adequate and well-controlled studies with KONVOMEP in pregnant women. KONVOMEP contains omeprazole and sodium bicarbonate.
There are no adequate and well-controlled studies with omeprazole in pregnant women. Available epidemiologic data fail to demonstrate an increased risk of major congenital malformations or other adverse pregnancy outcomes with first trimester omeprazole use (
There are no adequate and well-controlled studies with KONVOMEP in pregnant women. Four published epidemiological studies compared the frequency of congenital abnormalities among infants born to women who used omeprazole during pregnancy with the frequency of abnormalities among infants of women exposed to H2‑receptor antagonists or other controls.
A population-based retrospective cohort epidemiological study from the Swedish Medical Birth Register, covering approximately 99% of pregnancies, from 1995 to 99, reported on 955 infants (824 exposed during the first trimester with 39 of these exposed beyond first trimester, and 131 exposed after the first trimester) whose mothers used omeprazole during pregnancy. The number of infants exposed
A population-based retrospective cohort study covering all live births in Denmark from 1996 to 2009 reported on 1,800 live births whose mothers used omeprazole during the first trimester of pregnancy and 837,317 live births whose mothers did not use any PPI. The overall rate of birth defects in infants born to mothers with first trimester exposure to omeprazole was 2.9% and 2.6% in infants born to mothers not exposed to any PPI during the first trimester.
A retrospective cohort study reported on 689 pregnant women exposed to either H2‑blockers or omeprazole in the first trimester (134 exposed to omeprazole) and 1,572 pregnant women unexposed to either during the first trimester. The overall malformation rate in offspring born to mothers with first trimester exposure to omeprazole, an H2‑blocker, or were unexposed was 3.6%, 5.5%, and 4.1%, respectively.
A small prospective observational cohort study followed 113 women exposed to omeprazole during pregnancy (89% first trimester exposures). The reported rate of major congenital malformations was 4% in the omeprazole group, 2% in controls exposed to non-teratogens, and 2.8% in disease-paired controls. Rates of spontaneous and elective abortions, preterm deliveries, gestational age at delivery, and mean birth weight were similar among the groups.
Several studies have reported no apparent adverse short-term effects on the infant when single-dose oral or intravenous omeprazole was administered to over 200 pregnant women as premedication for cesarean section under general anesthesia.
Reproductive studies conducted with omeprazole in rats at oral doses up to 138 mg/kg/day (about 34 times an oral human dose of 40 mg on a body surface area basis) and in rabbits at doses up to 69.1 mg/kg/day (about 34 times an oral human dose of 40 mg on a body surface area basis) during organogenesis did not disclose any evidence for a teratogenic potential of omeprazole. In rabbits, omeprazole in a dose range of 6.9 to 69.1 mg/kg/day (about 3.4 to 34 times an oral human dose of 40 mg on a body surface area basis) administered during organogenesis produced dose-related increases in embryo-lethality, fetal resorptions, and pregnancy disruptions. In rats, dose-related embryo/fetal toxicity and postnatal developmental toxicity were observed in offspring resulting from parents treated with omeprazole at 13.8 to 138.0 mg/kg/day (about 3.4 to 34 times an oral human dose of 40 mg on a body surface area basis), administered prior to mating through the lactation period.
The data described below was generated from studies using esomeprazole, an enantiomer of omeprazole. The animal to human dose multiples are based on the assumption of equal systemic exposure to esomeprazole in humans following oral administration of either 40 mg esomeprazole or 40 mg omeprazole.
No effects on embryo-fetal development were observed in reproduction studies with esomeprazole magnesium in rats at oral doses up to 280 mg/kg/day (about 68 times an oral human dose of 40 mg on a body surface area basis) and in rabbits at oral doses up to 86 mg/kg/day (about 42 times an oral human dose of 40 mg of esomeprazole or 40 mg omeprazole on a body surface area basis) administered during organogenesis.
A pre- and postnatal developmental toxicity study in rats with additional endpoints to evaluate bone development were performed with esomeprazole magnesium at oral doses of 14 to 280 mg/kg/day (about 3.4 to 68 times an oral human dose of 40 mg of esomeprazole or 40 mg omeprazole on a body surface area basis). Neonatal/early postnatal (birth to weaning) survival was decreased at doses equal to or greater than 138 mg/kg/day (about 34 times an oral human dose of 40 mg esomeprazole or 40 mg omeprazole on a body surface area basis). Body weight and body weight gain were reduced and neurobehavioral or general developmental delays in the immediate post-weaning timeframe were evident at doses equal to or greater than 69 mg/kg/day (about 17 times an oral human dose of 40 mg esomeprazole or 40 mg omeprazole on a body surface area basis). In addition, decreased femur length, width and thickness of cortical bone, decreased thickness of the tibial growth plate and minimal to mild bone marrow hypocellularity were noted at doses of esomeprazole magnesium equal to or greater than 14 mg/kg/day (about 3.4 times an oral human dose of 40 mg esomeprazole or 40 mg omeprazole on a body surface area basis). Physeal dysplasia in the femur was observed in offspring of rats treated with oral doses of esomeprazole magnesium at doses equal to or greater than 138 mg/kg/day (about 34 times an oral human dose of 40 mg esomeprazole or 40 mg omeprazole on a body surface area basis).
Effects on maternal bone were observed in pregnant and lactating rats in a pre- and postnatal toxicity study when esomeprazole magnesium was administered at oral doses of 14 to 280 mg/kg/day (about 3.4 to 68 times an oral human dose of 40 mg esomeprazole or 40 mg omeprazole on a body surface area basis). When rats were dosed from gestational Day 7 through weaning on postnatal Day 21, a statistically significant decrease in maternal femur weight of up to 14% (as compared to placebo treatment) was observed at doses of esomeprazole magnesium equal to or greater than 138 mg/kg/day (about 34 times an oral human dose of 40 mg on a body surface area basis).
A pre- and postnatal development study in rats with esomeprazole strontium (using equimolar doses compared to esomeprazole magnesium study) produced similar results in dams and pups as described above.
A follow up developmental toxicity study in rats with further time points to evaluate pup bone development from postnatal Day 2 to adulthood was performed with esomeprazole magnesium at oral doses of 280 mg/kg/day (about 68 times an oral human dose of 40 mg on a body surface area basis) where esomeprazole administration was from either gestational Day 7 or gestational Day 16 until parturition. When maternal administration was confined to gestation only, there were no effects on bone physeal morphology in the offspring at any age.
Teratogenicity was not observed in animal reproduction studies with administration of oral esomeprazole (an enantiomer of omeprazole) magnesium in rats and rabbits during organogenesis with doses about 68‑times and 42‑times, respectively, an oral human dose of 40‑mg esomeprazole or 40 mg omeprazole (based on body surface area for a 60‑kg person). Changes in bone morphology were observed in offspring of rats dosed through most of pregnancy and lactation at doses equal to or greater than approximately 34-times an oral human dose of 40‑mg esomeprazole or 40‑mg omeprazole. When maternal administration was confined to gestation only, there were no effects on bone physeal morphology in the offspring at any age (
There are no adequate and well-controlled studies with KONVOMEP in pregnant women. Four published epidemiological studies compared the frequency of congenital abnormalities among infants born to women who used omeprazole during pregnancy with the frequency of abnormalities among infants of women exposed to H2‑receptor antagonists or other controls.
A population-based retrospective cohort epidemiological study from the Swedish Medical Birth Register, covering approximately 99% of pregnancies, from 1995 to 99, reported on 955 infants (824 exposed during the first trimester with 39 of these exposed beyond first trimester, and 131 exposed after the first trimester) whose mothers used omeprazole during pregnancy. The number of infants exposed
A population-based retrospective cohort study covering all live births in Denmark from 1996 to 2009 reported on 1,800 live births whose mothers used omeprazole during the first trimester of pregnancy and 837,317 live births whose mothers did not use any PPI. The overall rate of birth defects in infants born to mothers with first trimester exposure to omeprazole was 2.9% and 2.6% in infants born to mothers not exposed to any PPI during the first trimester.
A retrospective cohort study reported on 689 pregnant women exposed to either H2‑blockers or omeprazole in the first trimester (134 exposed to omeprazole) and 1,572 pregnant women unexposed to either during the first trimester. The overall malformation rate in offspring born to mothers with first trimester exposure to omeprazole, an H2‑blocker, or were unexposed was 3.6%, 5.5%, and 4.1%, respectively.
A small prospective observational cohort study followed 113 women exposed to omeprazole during pregnancy (89% first trimester exposures). The reported rate of major congenital malformations was 4% in the omeprazole group, 2% in controls exposed to non-teratogens, and 2.8% in disease-paired controls. Rates of spontaneous and elective abortions, preterm deliveries, gestational age at delivery, and mean birth weight were similar among the groups.
Several studies have reported no apparent adverse short-term effects on the infant when single-dose oral or intravenous omeprazole was administered to over 200 pregnant women as premedication for cesarean section under general anesthesia.
Reproductive studies conducted with omeprazole in rats at oral doses up to 138 mg/kg/day (about 34 times an oral human dose of 40 mg on a body surface area basis) and in rabbits at doses up to 69.1 mg/kg/day (about 34 times an oral human dose of 40 mg on a body surface area basis) during organogenesis did not disclose any evidence for a teratogenic potential of omeprazole. In rabbits, omeprazole in a dose range of 6.9 to 69.1 mg/kg/day (about 3.4 to 34 times an oral human dose of 40 mg on a body surface area basis) administered during organogenesis produced dose-related increases in embryo-lethality, fetal resorptions, and pregnancy disruptions. In rats, dose-related embryo/fetal toxicity and postnatal developmental toxicity were observed in offspring resulting from parents treated with omeprazole at 13.8 to 138.0 mg/kg/day (about 3.4 to 34 times an oral human dose of 40 mg on a body surface area basis), administered prior to mating through the lactation period.
The data described below was generated from studies using esomeprazole, an enantiomer of omeprazole. The animal to human dose multiples are based on the assumption of equal systemic exposure to esomeprazole in humans following oral administration of either 40 mg esomeprazole or 40 mg omeprazole.
No effects on embryo-fetal development were observed in reproduction studies with esomeprazole magnesium in rats at oral doses up to 280 mg/kg/day (about 68 times an oral human dose of 40 mg on a body surface area basis) and in rabbits at oral doses up to 86 mg/kg/day (about 42 times an oral human dose of 40 mg of esomeprazole or 40 mg omeprazole on a body surface area basis) administered during organogenesis.
A pre- and postnatal developmental toxicity study in rats with additional endpoints to evaluate bone development were performed with esomeprazole magnesium at oral doses of 14 to 280 mg/kg/day (about 3.4 to 68 times an oral human dose of 40 mg of esomeprazole or 40 mg omeprazole on a body surface area basis). Neonatal/early postnatal (birth to weaning) survival was decreased at doses equal to or greater than 138 mg/kg/day (about 34 times an oral human dose of 40 mg esomeprazole or 40 mg omeprazole on a body surface area basis). Body weight and body weight gain were reduced and neurobehavioral or general developmental delays in the immediate post-weaning timeframe were evident at doses equal to or greater than 69 mg/kg/day (about 17 times an oral human dose of 40 mg esomeprazole or 40 mg omeprazole on a body surface area basis). In addition, decreased femur length, width and thickness of cortical bone, decreased thickness of the tibial growth plate and minimal to mild bone marrow hypocellularity were noted at doses of esomeprazole magnesium equal to or greater than 14 mg/kg/day (about 3.4 times an oral human dose of 40 mg esomeprazole or 40 mg omeprazole on a body surface area basis). Physeal dysplasia in the femur was observed in offspring of rats treated with oral doses of esomeprazole magnesium at doses equal to or greater than 138 mg/kg/day (about 34 times an oral human dose of 40 mg esomeprazole or 40 mg omeprazole on a body surface area basis).
Effects on maternal bone were observed in pregnant and lactating rats in a pre- and postnatal toxicity study when esomeprazole magnesium was administered at oral doses of 14 to 280 mg/kg/day (about 3.4 to 68 times an oral human dose of 40 mg esomeprazole or 40 mg omeprazole on a body surface area basis). When rats were dosed from gestational Day 7 through weaning on postnatal Day 21, a statistically significant decrease in maternal femur weight of up to 14% (as compared to placebo treatment) was observed at doses of esomeprazole magnesium equal to or greater than 138 mg/kg/day (about 34 times an oral human dose of 40 mg on a body surface area basis).
A pre- and postnatal development study in rats with esomeprazole strontium (using equimolar doses compared to esomeprazole magnesium study) produced similar results in dams and pups as described above.
A follow up developmental toxicity study in rats with further time points to evaluate pup bone development from postnatal Day 2 to adulthood was performed with esomeprazole magnesium at oral doses of 280 mg/kg/day (about 68 times an oral human dose of 40 mg on a body surface area basis) where esomeprazole administration was from either gestational Day 7 or gestational Day 16 until parturition. When maternal administration was confined to gestation only, there were no effects on bone physeal morphology in the offspring at any age.
Available data with sodium bicarbonate use in pregnant women have not identified a drug associated risk of major birth defects or miscarriage. Published animal studies report that sodium bicarbonate administered to rats, mice or rabbits during pregnancy did not cause adverse developmental effects in offspring.
The estimated background risks of major birth defects and miscarriage for the indicated population are unknown. All pregnancies have a background risk of birth defect, loss or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
KONVOMEP is contraindicated in patients with known hypersensitivity to substituted benzimidazoles or to any components of the formulation. Hypersensitivity reactions may include anaphylaxis, anaphylactic shock, angioedema, bronchospasm, acute tubulointerstitial nephritis, and urticaria
Acute tubulointerstitial nephritis (TIN) has been observed in patients taking PPIs and may occur at any point during PPI therapy. Patients may present with varying signs and symptoms from symptomatic hypersensitivity reactions to non-specific symptoms of decreased renal function (e.g., malaise, nausea, anorexia). In reported case series, some patients were diagnosed on biopsy and in the absence of extra-renal manifestations (e.g., fever, rash or arthralgia).
Discontinue KONVOMEP and evaluate patients with suspected acute TIN
The following adverse reactions have been identified during post-approval use of omeprazole and sodium bicarbonate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Metabolic alkalosis, seizures, and tetany.
Proton pump inhibitors (PPIs), including KONVOMEP, are contraindicated in patients receiving rilpivirine containing products
Table 5and Table 6include drugs with clinically important drug interactions and interaction with diagnostics when administered concomitantly with omeprazole and instructions for preventing or managing them.
Consult the labeling of concomitantly used drugs to obtain further information about interactions with PPIs.
Antiretrovirals | |
Clinical Impact: | The effect of PPIs on antiretroviral drugs is variable. The clinical importance and the mechanisms behind these interactions are not always known.
|
Intervention: | Rilpivirine-containing products : Concomitant use with KONVOMEP is contraindicated[see Contraindications ] .Atazanavir : Avoid concomitant use with KONVOMEP. See prescribing information for atazanavir for dosing information.Nelfinavir : Avoid concomitant use with KONVOMEP. See prescribing information for nelfinavir.Saquinavir : See the prescribing information for saquinavir for monitoring of potential saquinavir-related toxicities.Other antiretrovirals : See prescribing information for specific antiretroviral drugs. |
Warfarin | |
Clinical Impact: | Increased INR and prothrombin time in patients receiving PPIs, including omeprazole, and warfarin concomitantly. Increases in INR and prothrombin time may lead to abnormal bleeding and even death. |
Intervention: | Monitor INR and prothrombin time and adjust the dose of warfarin, if needed, to maintain target INR range. |
Methotrexate | |
Clinical Impact: | Concomitant use of omeprazole with methotrexate (primarily at high dose) may elevate and prolong serum concentrations of methotrexate and/or its metabolite hydroxymethotrexate, possibly leading to methotrexate toxicities. No formal drug interaction studies of high-dose methotrexate with PPIs have been conducted [see Warnings and Precautions ] . |
Intervention: | A temporary withdrawal of KONVOMEP may be considered in some patients receiving high‑dose methotrexate. |
CYP2C19 Substrates (e.g., clopidogrel, citalopram, cilostazol, phenytoin, diazepam) | |
Clopidogrel | |
Clinical Impact: | Concomitant use of omeprazole 80 mg results in reduced plasma concentrations of the active metabolite of clopidogrel and a reduction in platelet inhibition [see Clinical Pharmacology ] . |
There are no adequate combination studies of a lower dose of omeprazole or a higher dose of clopidogrel in comparison with the approved dose of clopidogrel. | |
Intervention: | Avoid concomitant use with KONVOMEP. Consider use of alternative anti-platelet therapy [see Warnings and Precautions ] . |
Citalopram | |
Clinical Impact: | Increased exposure of citalopram leading to an increased risk of QT prolongation [see Clinical Pharmacology ] . |
Intervention: | Limit the dose of citalopram to a maximum of 20 mg per day. See prescribing information for citalopram. |
Cilostazol | |
Clinical Impact: | Increased exposure of one of the active metabolites of cilostazol (3,4-dihydro-cilostazol) [see Clinical Pharmacology ] . |
Intervention: | Reduce the dose of cilostazol to 50 mg twice daily. See prescribing information for cilostazol. |
Phenytoin | |
Clinical Impact: | Potential for increased exposure of phenytoin. |
Intervention: | Monitor phenytoin serum concentrations. Dose adjustment may be needed to maintain therapeutic drug concentrations. See prescribing information for phenytoin. |
Diazepam | |
Clinical Impact: | Increased exposure of diazepam [see Clinical Pharmacology ] . |
Intervention: | Monitor patients for increased sedation and reduce the dose of diazepam as needed. |
Digoxin | |
Clinical Impact: | Potential for increased exposure of digoxin [see Clinical Pharmacology ] . |
Intervention: | Monitor digoxin concentrations. Dose adjustment may be needed to maintain therapeutic drug concentrations. See digoxin prescribing information. |
Drugs Dependent on Gastric pH for Absorption (e.g., iron salts, erlotinib, dasatinib, nilotinib, mycophenolate mofetil, ketoconazole/itraconazole) | |
Clinical Impact: | Omeprazole can reduce the absorption of other drugs due to its effect on reducing intragastric acidity. |
Intervention: | Mycophenolate mofetil (MMF) : Co-administration of omeprazole in healthy subjects and in transplant patients receiving MMF has been reported to reduce the exposure to the active metabolite, mycophenolic acid (MPA), possibly due to a decrease in MMF solubility at an increased gastric pH. The clinical relevance of reduced MPA exposure on organ rejection has not been established in transplant patients receiving KONVOMEP and MMF. Use KONVOMEP with caution in transplant patients receiving MMF[see Clinical Pharmacology ] .See the prescribing information for other drugs dependent on gastric pH for absorption. |
Tacrolimus | |
Clinical Impact: | Potential for increased exposure of tacrolimus, especially in transplant patients who are intermediate or poor metabolizers of CYP2C19. |
Intervention: | Monitor tacrolimus whole blood concentrations. Dose adjustment may be needed to maintain therapeutic drug concentrations. See prescribing information for tacrolimus. |
Interactions with Investigations of Neuroendocrine Tumors | |
Clinical Impact: | Serum chromogranin A (CgA) levels increase secondary to PPI-induced decreases in gastric acidity. The increased CgA level may cause false positive results in diagnostic investigations for neuroendocrine tumors [see Warnings and Precautions and Clinical Pharmacology ] . |
Intervention: | Temporarily stop KONVOMEP treatment at least 14 days before assessing CgA levels and consider repeating the test if initial CgA levels are high. If serial tests are performed (e.g., for monitoring), the same commercial laboratory should be used for testing, as reference ranges between tests may vary. |
Interaction with Secretin Stimulation Test | |
Clinical Impact: | Hyper-response in gastrin secretion in response to secretin stimulation test, falsely suggesting gastrinoma. |
Intervention: | Temporarily stop KONVOMEP treatment at least 14 days before assessing to allow gastrin levels to return to baseline [see Clinical Pharmacology ] . |
False Positive Urine Tests for THC | |
Clinical Impact: | There have been reports of false positive urine screening tests for tetrahydrocannabinol (THC) in patients receiving PPIs. |
Intervention: | An alternative confirmatory method should be considered to verify positive results. |
Other | |
Clinical Impact: | There have been clinical reports of interactions with other drugs metabolized via the cytochrome P450 system (e.g., cyclosporine, disulfiram). |
Intervention: | Monitor patients to determine if it is necessary to adjust the dosage of these other drugs when taken concomitantly with KONVOMEP. |
CYP2C19 or CYP3A4 Inducers | |
Clinical Impact: | Decreased exposure of omeprazole when used concomitantly with strong inducers [see Clinical Pharmacology ] . |
Intervention: | St. John’s Wort, rifampin : Avoid concomitant use with KONVOMEP[see Warnings and Precautions ] .Ritonavir-containing products : see prescribing information for specific drugs. |
CYP2C19 or CYP3A4 Inhibitors | |
Clinical Impact: | Increased exposure of omeprazole [see Clinical Pharmacology ] . |
Intervention: | Voriconazole : Dosage adjustment of KONVOMEP is not usually required. See full prescribing information for voriconazole. |
See Full Prescribing Information for a list of clinically important drug interactions.
- Gastric Malignancy: In adults, symptomatic response does not preclude the presence of gastric malignancy. Consider additional follow-up and diagnostic testing. ()
5.1 Presence of Gastric MalignancyIn adults, symptomatic response to therapy with KONVOMEP does not preclude the presence of gastric malignancy. Consider additional follow-up and diagnostic testing in adult patients who have a suboptimal response or an early symptomatic relapse after completing treatment with a proton pump inhibitor (PPI). In older patients, also consider an endoscopy.
- Acute Tubulointerstitial Nephritis: Discontinue treatment and evaluate patients. ()
5.2 Acute Tubulointerstitial NephritisAcute tubulointerstitial nephritis (TIN) has been observed in patients taking PPIs and may occur at any point during PPI therapy. Patients may present with varying signs and symptoms from symptomatic hypersensitivity reactions to non-specific symptoms of decreased renal function (e.g., malaise, nausea, anorexia). In reported case series, some patients were diagnosed on biopsy and in the absence of extra-renal manifestations (e.g., fever, rash or arthralgia).
Discontinue KONVOMEP and evaluate patients with suspected acute TIN
[see Contraindications ]. - Sodium Content: Take sodium content into consideration in patients on a sodium-restricted diet. Avoid in patients with Bartter’s syndrome, hypokalemia, hypocalcemia, and problems with acid-base balance. ()
5.3 Sodium ContentEach mL of reconstituted KONVOMEP contains 84 mg of sodium bicarbonate (equivalent to 1 mEq/mL of sodium). The total content of sodium, from active and inactive ingredients per mL of reconstituted KONVOMEP is 26.3 mg (1.14 mEq). Total sodium content per 40 mg dose (volume of 20 mL) of KONVOMEP is 526 mg (22.8 mEq).
Chronic administration of bicarbonate with calcium or milk can cause milk-alkali syndrome. Chronic use of sodium bicarbonate may lead to systemic alkalosis, and increased sodium intake can produce edema and weight gain.
The sodium content of KONVOMEP should be taken into consideration when administering to patients on a sodium-restricted diet or those at risk for developing congestive heart failure.
Avoid KONVOMEP in patients with Bartter’s syndrome, hypokalemia, hypocalcemia, and problems with acid‑base balance.
- : PPI therapy may be associated with increased risk. (Clostridium difficile-Associated Diarrhea)
5.4Clostridium difficile-Associated DiarrheaPublished observational studies suggest that PPI therapy like KONVOMEP may be associated with an increased risk of
Clostridium difficile-associated diarrhea, especially in hospitalized patients. This diagnosis should be considered for diarrhea that does not improve[see Adverse Reactions ].Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated.
- Bone Fracture: Long-term and multiple daily dose PPI therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist, or spine. ()
5.5 Bone FractureSeveral published observational studies suggest that PPI therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist, or spine. The risk of fracture was increased in patients who received high-dose, defined as multiple daily doses, and long-term PPI therapy (a year or longer). Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated. Patients at risk for osteoporosis-related fractures should be managed according to the established treatment guidelines
[see Dosage and Administration and Adverse Reactions ]. - Severe Cutaneous Adverse Reactions: Discontinue at the first signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation. ()
5.6 Severe Cutaneous Adverse ReactionsSevere cutaneous adverse reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) have been reported in association with the use of PPIs
[see Adverse Reactions ]. Discontinue KONVOMEP at the first signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation. - Cutaneous and Systemic Lupus Erythematosus: Mostly cutaneous; new onset or exacerbation of existing disease; discontinue KONVOMEP and refer to specialist for evaluation. ()
5.7 Cutaneous and Systemic Lupus ErythematosusCutaneous lupus erythematosus (CLE) and systemic lupus erythematosus (SLE) have been reported in patients taking PPIs, including omeprazole. These events have occurred as both new onset and an exacerbation of existing autoimmune disease. The majority of PPI-induced lupus erythematous cases were CLE.
The most common form of CLE reported in patients treated with PPIs was subacute CLE (SCLE) and occurred within weeks to years after continuous drug therapy in patients ranging from infants to the elderly. Generally, histological findings were observed without organ involvement.
Systemic lupus erythematosus (SLE) is less commonly reported than CLE in patients receiving PPIs. PPI associated SLE is usually milder than non-drug induced SLE. Onset of SLE typically occurred within days to years after initiating treatment in patients ranging from young adults to the elderly. The majority of patients presented with rash; however, arthralgia and cytopenia were also reported.
Avoid administration of PPIs for longer than medically indicated. If signs or symptoms consistent with CLE or SLE are noted in patients receiving KONVOMEP, discontinue the drug and refer the patient to the appropriate specialist for evaluation. Most patients improve with discontinuation of the PPI alone in 4 to 12 weeks. Serological testing (e.g., ANA) may be positive and elevated serological test results may take longer to resolve than clinical manifestations.
- Interaction with Clopidogrel: Avoid concomitant use of KONVOMEP. ()
5.8 Interaction with ClopidogrelAvoid concomitant use of KONVOMEP with clopidogrel. Clopidogrel is a prodrug. Inhibition of platelet aggregation by clopidogrel is entirely due to an active metabolite. The metabolism of clopidogrel to its active metabolite can be impaired by use with concomitant medications, such as omeprazole, that interfere with CYP2C19 activity. Concomitant use of clopidogrel with 80 mg omeprazole reduces the pharmacological activity of clopidogrel, even when administered 12 hours apart. When using KONVOMEP, consider alternative antiplatelet therapy
[see Drug Interactions and Clinical Pharmacology ]. - Cyanocobalamin (Vitamin B-12) Deficiency: Daily long-term use (e.g., longer than 3 years) may lead to malabsorption or a deficiency of cyanocobalamin. ()
5.9 Cyanocobalamin (Vitamin B-12) DeficiencyDaily treatment with any acid-suppressing medications over a long period of time (e.g., longer than 3 years) may lead to malabsorption of cyanocobalamin (vitamin B-12) caused by hypo or achlorhydria. Rare reports of cyanocobalamin deficiency occurring with acid-suppressing therapy have been reported in the literature. This diagnosis should be considered if clinical symptoms consistent with cyanocobalamin deficiency are observed in patients treated with KONVOMEP.
- Hypomagnesemia and Mineral Metabolism: Reported rarely with prolonged treatment with PPIs. ()
5.10 Hypomagnesemia and Mineral MetabolismHypomagnesemia, symptomatic and asymptomatic, has been reported rarely in patients treated with PPIs for at least three months, in most cases after a year of therapy. Serious adverse events include tetany, arrhythmias, and seizures.
Hypomagnesemia may lead to hypocalcemia and/or hypokalemia and may exacerbate underlying hypocalcemia in at-risk patients. In most patients, treatment of hypomagnesemia required magnesium replacement and discontinuation of the PPI.
For patients expected to be on prolonged treatment or who take PPIs with medications such as digoxin or drugs that may cause hypomagnesemia (e.g., diuretics), health care professionals may consider monitoring magnesium levels prior to initiation of PPI treatment and periodically
[see Adverse Reactions ].Consider monitoring magnesium and calcium levels prior to initiation of KONVOMEP and periodically while on treatment in patients with a preexisting risk of hypocalcemia (e.g., hypoparathyroidism). Supplement with magnesium and/or calcium as necessary. If hypocalcemia is refractory to treatment, consider discontinuing the PPI.
- Interaction with St. John’s Wort or Rifampin: Avoid concomitant use of KONVOMEP. (,
5.11 Interaction with St. John’s Wort or RifampinDrugs which induce CYP2C19 or CYP3A4 (such as St. John’s wort or rifampin) can substantially decrease omeprazole concentrations
[see Drug Interactions ]. Avoid concomitant use of KONVOMEP with St. John’s wort or rifampin.)7 DRUG INTERACTIONSTable 5and Table 6include drugs with clinically important drug interactions and interaction with diagnostics when administered concomitantly with omeprazole and instructions for preventing or managing them.
Consult the labeling of concomitantly used drugs to obtain further information about interactions with PPIs.
Table 5: Clinically Relevant Interactions Affecting Drugs Co-Administered with Omeprazole and Interaction with Diagnostics AntiretroviralsClinical Impact:The effect of PPIs on antiretroviral drugs is variable. The clinical importance and the mechanisms behind these interactions are not always known.
- Decreased exposure of some antiretroviral drugs (e.g., rilpivirine, atazanavir and nelfinavir) when used concomitantly with omeprazole may reduce antiviral effect and promote the development of drug resistance[see Clinical Pharmacology ].
- Increased exposure of other antiretroviral drugs (e.g., saquinavir) when used concomitantly with omeprazole may increase toxicity[see Clinical Pharmacology ].
- There are other antiretroviral drugs which do not result in clinically relevant interactions with omeprazole.
Intervention:Rilpivirine-containing products: Concomitant use with KONVOMEP is contraindicated[see Contraindications ].Atazanavir: Avoid concomitant use with KONVOMEP. See prescribing information for atazanavir for dosing information.Nelfinavir: Avoid concomitant use with KONVOMEP. See prescribing information for nelfinavir.Saquinavir: See the prescribing information for saquinavir for monitoring of potential saquinavir-related toxicities.Other antiretrovirals: See prescribing information for specific antiretroviral drugs.WarfarinClinical Impact:Increased INR and prothrombin time in patients receiving PPIs, including omeprazole, and warfarin concomitantly. Increases in INR and prothrombin time may lead to abnormal bleeding and even death.
Intervention:Monitor INR and prothrombin time and adjust the dose of warfarin, if needed, to maintain target INR range.
MethotrexateClinical Impact:Concomitant use of omeprazole with methotrexate (primarily at high dose) may elevate and prolong serum concentrations of methotrexate and/or its metabolite hydroxymethotrexate, possibly leading to methotrexate toxicities. No formal drug interaction studies of high-dose methotrexate with PPIs have been conducted
[see Warnings and Precautions ].Intervention:A temporary withdrawal of KONVOMEP may be considered in some patients receiving high‑dose methotrexate.
CYP2C19 Substrates (e.g., clopidogrel, citalopram, cilostazol, phenytoin, diazepam)Clopidogrel
Clinical Impact:Concomitant use of omeprazole 80 mg results in reduced plasma concentrations of the active metabolite of clopidogrel and a reduction in platelet inhibition
[see Clinical Pharmacology ].There are no adequate combination studies of a lower dose of omeprazole or a higher dose of clopidogrel in comparison with the approved dose of clopidogrel.
Intervention:Avoid concomitant use with KONVOMEP. Consider use of alternative anti-platelet therapy
[see Warnings and Precautions ].Citalopram
Clinical Impact:Increased exposure of citalopram leading to an increased risk of QT prolongation
[see Clinical Pharmacology ].Intervention:Limit the dose of citalopram to a maximum of 20 mg per day. See prescribing information for citalopram.
Cilostazol
Clinical Impact:Increased exposure of one of the active metabolites of cilostazol (3,4-dihydro-cilostazol)
[see Clinical Pharmacology ].Intervention:Reduce the dose of cilostazol to 50 mg twice daily. See prescribing information for cilostazol.
Phenytoin
Clinical Impact:Potential for increased exposure of phenytoin.
Intervention:Monitor phenytoin serum concentrations. Dose adjustment may be needed to maintain therapeutic drug concentrations. See prescribing information for phenytoin.
Diazepam
Clinical Impact:Increased exposure of diazepam
[see Clinical Pharmacology ].Intervention:Monitor patients for increased sedation and reduce the dose of diazepam as needed.
DigoxinClinical Impact:Potential for increased exposure of digoxin
[see Clinical Pharmacology ].Intervention:Monitor digoxin concentrations. Dose adjustment may be needed to maintain therapeutic drug concentrations. See digoxin prescribing information.
Drugs Dependent on Gastric pH for Absorption (e.g., iron salts, erlotinib, dasatinib, nilotinib, mycophenolate mofetil, ketoconazole/itraconazole)Clinical Impact:Omeprazole can reduce the absorption of other drugs due to its effect on reducing intragastric acidity.
Intervention:Mycophenolate mofetil (MMF): Co-administration of omeprazole in healthy subjects and in transplant patients receiving MMF has been reported to reduce the exposure to the active metabolite, mycophenolic acid (MPA), possibly due to a decrease in MMF solubility at an increased gastric pH. The clinical relevance of reduced MPA exposure on organ rejection has not been established in transplant patients receiving KONVOMEP and MMF. Use KONVOMEP with caution in transplant patients receiving MMF[see Clinical Pharmacology ].See the prescribing information for other drugs dependent on gastric pH for absorption.
TacrolimusClinical Impact:Potential for increased exposure of tacrolimus, especially in transplant patients who are intermediate or poor metabolizers of CYP2C19.
Intervention:Monitor tacrolimus whole blood concentrations. Dose adjustment may be needed to maintain therapeutic drug concentrations. See prescribing information for tacrolimus.
Interactions with Investigations of Neuroendocrine TumorsClinical Impact:Serum chromogranin A (CgA) levels increase secondary to PPI-induced decreases in gastric acidity. The increased CgA level may cause false positive results in diagnostic investigations for neuroendocrine tumors
[see Warnings and Precautions and Clinical Pharmacology ].Intervention:Temporarily stop KONVOMEP treatment at least 14 days before assessing CgA levels and consider repeating the test if initial CgA levels are high. If serial tests are performed (e.g., for monitoring), the same commercial laboratory should be used for testing, as reference ranges between tests may vary.
Interaction with Secretin Stimulation TestClinical Impact:Hyper-response in gastrin secretion in response to secretin stimulation test, falsely suggesting gastrinoma.
Intervention:Temporarily stop KONVOMEP treatment at least 14 days before assessing to allow gastrin levels to return to baseline
[see Clinical Pharmacology ].False Positive Urine Tests for THCClinical Impact:There have been reports of false positive urine screening tests for tetrahydrocannabinol (THC) in patients receiving PPIs.
Intervention:An alternative confirmatory method should be considered to verify positive results.
OtherClinical Impact:There have been clinical reports of interactions with other drugs metabolized via the cytochrome P450 system (e.g., cyclosporine, disulfiram).
Intervention:Monitor patients to determine if it is necessary to adjust the dosage of these other drugs when taken concomitantly with KONVOMEP.
Table 6: Clinically Relevant Interactions Affecting Omeprazole when Co-Administered with Other Drugs CYP2C19 or CYP3A4 InducersClinical Impact:Decreased exposure of omeprazole when used concomitantly with strong inducers
[see Clinical Pharmacology ].Intervention:St. John’s Wort, rifampin: Avoid concomitant use with KONVOMEP[see Warnings and Precautions ].Ritonavir-containing products: see prescribing information for specific drugs.CYP2C19 or CYP3A4 InhibitorsClinical Impact:Increased exposure of omeprazole
[see Clinical Pharmacology ].Intervention:Voriconazole: Dosage adjustment of KONVOMEP is not usually required. See full prescribing information for voriconazole.See Full Prescribing Information for a list of clinically important drug interactions.
- Decreased exposure of some antiretroviral drugs (e.g., rilpivirine, atazanavir and nelfinavir) when used concomitantly with omeprazole may reduce antiviral effect and promote the development of drug resistance
- Interactions with Diagnostic Investigations for Neuroendocrine Tumors: Increased Chromogranin A (CgA) levels may interfere with diagnostic investigations for neuroendocrine tumors; temporarily stop KONVOMEP at least 14 days before assessing CgA levels. ()
5.12 Interactions with Investigations for Neuroendocrine TumorsSerum chromogranin A (CgA) levels increase secondary to drug-induced decreases in gastric acidity. The increased CgA level may cause false positive results in diagnostic investigations for neuroendocrine tumors. Providers should temporarily stop KONVOMEP treatment for at least 14 days before assessing CgA levels and consider repeating the test if initial CgA levels are high. If serial tests are performed (e.g., for monitoring), the same commercial laboratory should be used for testing, as reference ranges between tests may vary
[see Drug Interactions ]. - Interaction with Methotrexate: Concomitant use with PPIs may elevate and/or prolong serum concentrations of methotrexate and/or its metabolite, possibly leading to toxicity. With high dose methotrexate administration, consider a temporary withdrawal of KONVOMEP. (,
5.13 Interaction with MethotrexateLiterature suggests that concomitant use of PPIs with methotrexate (primarily at high-dose) may elevate and prolong serum levels of methotrexate and/or its metabolite, possibly leading to methotrexate toxicities. In high‑dose methotrexate administration, a temporary withdrawal of the PPI may be considered in some patients
[see Drug Interactions ].)7 DRUG INTERACTIONSTable 5and Table 6include drugs with clinically important drug interactions and interaction with diagnostics when administered concomitantly with omeprazole and instructions for preventing or managing them.
Consult the labeling of concomitantly used drugs to obtain further information about interactions with PPIs.
Table 5: Clinically Relevant Interactions Affecting Drugs Co-Administered with Omeprazole and Interaction with Diagnostics AntiretroviralsClinical Impact:The effect of PPIs on antiretroviral drugs is variable. The clinical importance and the mechanisms behind these interactions are not always known.
- Decreased exposure of some antiretroviral drugs (e.g., rilpivirine, atazanavir and nelfinavir) when used concomitantly with omeprazole may reduce antiviral effect and promote the development of drug resistance[see Clinical Pharmacology ].
- Increased exposure of other antiretroviral drugs (e.g., saquinavir) when used concomitantly with omeprazole may increase toxicity[see Clinical Pharmacology ].
- There are other antiretroviral drugs which do not result in clinically relevant interactions with omeprazole.
Intervention:Rilpivirine-containing products: Concomitant use with KONVOMEP is contraindicated[see Contraindications ].Atazanavir: Avoid concomitant use with KONVOMEP. See prescribing information for atazanavir for dosing information.Nelfinavir: Avoid concomitant use with KONVOMEP. See prescribing information for nelfinavir.Saquinavir: See the prescribing information for saquinavir for monitoring of potential saquinavir-related toxicities.Other antiretrovirals: See prescribing information for specific antiretroviral drugs.WarfarinClinical Impact:Increased INR and prothrombin time in patients receiving PPIs, including omeprazole, and warfarin concomitantly. Increases in INR and prothrombin time may lead to abnormal bleeding and even death.
Intervention:Monitor INR and prothrombin time and adjust the dose of warfarin, if needed, to maintain target INR range.
MethotrexateClinical Impact:Concomitant use of omeprazole with methotrexate (primarily at high dose) may elevate and prolong serum concentrations of methotrexate and/or its metabolite hydroxymethotrexate, possibly leading to methotrexate toxicities. No formal drug interaction studies of high-dose methotrexate with PPIs have been conducted
[see Warnings and Precautions ].Intervention:A temporary withdrawal of KONVOMEP may be considered in some patients receiving high‑dose methotrexate.
CYP2C19 Substrates (e.g., clopidogrel, citalopram, cilostazol, phenytoin, diazepam)Clopidogrel
Clinical Impact:Concomitant use of omeprazole 80 mg results in reduced plasma concentrations of the active metabolite of clopidogrel and a reduction in platelet inhibition
[see Clinical Pharmacology ].There are no adequate combination studies of a lower dose of omeprazole or a higher dose of clopidogrel in comparison with the approved dose of clopidogrel.
Intervention:Avoid concomitant use with KONVOMEP. Consider use of alternative anti-platelet therapy
[see Warnings and Precautions ].Citalopram
Clinical Impact:Increased exposure of citalopram leading to an increased risk of QT prolongation
[see Clinical Pharmacology ].Intervention:Limit the dose of citalopram to a maximum of 20 mg per day. See prescribing information for citalopram.
Cilostazol
Clinical Impact:Increased exposure of one of the active metabolites of cilostazol (3,4-dihydro-cilostazol)
[see Clinical Pharmacology ].Intervention:Reduce the dose of cilostazol to 50 mg twice daily. See prescribing information for cilostazol.
Phenytoin
Clinical Impact:Potential for increased exposure of phenytoin.
Intervention:Monitor phenytoin serum concentrations. Dose adjustment may be needed to maintain therapeutic drug concentrations. See prescribing information for phenytoin.
Diazepam
Clinical Impact:Increased exposure of diazepam
[see Clinical Pharmacology ].Intervention:Monitor patients for increased sedation and reduce the dose of diazepam as needed.
DigoxinClinical Impact:Potential for increased exposure of digoxin
[see Clinical Pharmacology ].Intervention:Monitor digoxin concentrations. Dose adjustment may be needed to maintain therapeutic drug concentrations. See digoxin prescribing information.
Drugs Dependent on Gastric pH for Absorption (e.g., iron salts, erlotinib, dasatinib, nilotinib, mycophenolate mofetil, ketoconazole/itraconazole)Clinical Impact:Omeprazole can reduce the absorption of other drugs due to its effect on reducing intragastric acidity.
Intervention:Mycophenolate mofetil (MMF): Co-administration of omeprazole in healthy subjects and in transplant patients receiving MMF has been reported to reduce the exposure to the active metabolite, mycophenolic acid (MPA), possibly due to a decrease in MMF solubility at an increased gastric pH. The clinical relevance of reduced MPA exposure on organ rejection has not been established in transplant patients receiving KONVOMEP and MMF. Use KONVOMEP with caution in transplant patients receiving MMF[see Clinical Pharmacology ].See the prescribing information for other drugs dependent on gastric pH for absorption.
TacrolimusClinical Impact:Potential for increased exposure of tacrolimus, especially in transplant patients who are intermediate or poor metabolizers of CYP2C19.
Intervention:Monitor tacrolimus whole blood concentrations. Dose adjustment may be needed to maintain therapeutic drug concentrations. See prescribing information for tacrolimus.
Interactions with Investigations of Neuroendocrine TumorsClinical Impact:Serum chromogranin A (CgA) levels increase secondary to PPI-induced decreases in gastric acidity. The increased CgA level may cause false positive results in diagnostic investigations for neuroendocrine tumors
[see Warnings and Precautions and Clinical Pharmacology ].Intervention:Temporarily stop KONVOMEP treatment at least 14 days before assessing CgA levels and consider repeating the test if initial CgA levels are high. If serial tests are performed (e.g., for monitoring), the same commercial laboratory should be used for testing, as reference ranges between tests may vary.
Interaction with Secretin Stimulation TestClinical Impact:Hyper-response in gastrin secretion in response to secretin stimulation test, falsely suggesting gastrinoma.
Intervention:Temporarily stop KONVOMEP treatment at least 14 days before assessing to allow gastrin levels to return to baseline
[see Clinical Pharmacology ].False Positive Urine Tests for THCClinical Impact:There have been reports of false positive urine screening tests for tetrahydrocannabinol (THC) in patients receiving PPIs.
Intervention:An alternative confirmatory method should be considered to verify positive results.
OtherClinical Impact:There have been clinical reports of interactions with other drugs metabolized via the cytochrome P450 system (e.g., cyclosporine, disulfiram).
Intervention:Monitor patients to determine if it is necessary to adjust the dosage of these other drugs when taken concomitantly with KONVOMEP.
Table 6: Clinically Relevant Interactions Affecting Omeprazole when Co-Administered with Other Drugs CYP2C19 or CYP3A4 InducersClinical Impact:Decreased exposure of omeprazole when used concomitantly with strong inducers
[see Clinical Pharmacology ].Intervention:St. John’s Wort, rifampin: Avoid concomitant use with KONVOMEP[see Warnings and Precautions ].Ritonavir-containing products: see prescribing information for specific drugs.CYP2C19 or CYP3A4 InhibitorsClinical Impact:Increased exposure of omeprazole
[see Clinical Pharmacology ].Intervention:Voriconazole: Dosage adjustment of KONVOMEP is not usually required. See full prescribing information for voriconazole.See Full Prescribing Information for a list of clinically important drug interactions.
- Decreased exposure of some antiretroviral drugs (e.g., rilpivirine, atazanavir and nelfinavir) when used concomitantly with omeprazole may reduce antiviral effect and promote the development of drug resistance
- Fundic Gland Polyps: Risk increases with long-term use, especially beyond one year. Use the shortest duration of therapy. ()
5.14 Fundic Gland PolypsPPI use is associated with an increased risk of fundic gland polyps that increases with long-term use, especially beyond one year. Most PPI users who developed fundic gland polyps were asymptomatic and fundic gland polyps were identified incidentally on endoscopy. Use the shortest duration of PPI therapy appropriate to the condition being treated.