Get your patient on Koselugo (Selumetinib)
Koselugo prior authorization resources
Most recent state uniform prior authorization forms
Brand Resources
Koselugo patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
- KOSELUGO capsules: The recommended dosage is 25 mg/m 2 , swallowed whole, taken orally twice daily with or without food (see Table 1) . (2.1 , 2.2 )
- KOSELUGO oral granules: The recommended dosage is equivalent to 25 mg/m 2 , sprinkled onto or mixed with soft food and taken orally twice daily (see Table 2). (2.1 , 2.2 )
- Moderate hepatic impairment (Child-Pugh B): The recommended dosage is 20 mg/m 2 orally twice daily (see Tables 6 and 7) . (2.2 , 2.4 )
- Severe hepatic impairment (Child-Pugh C): The recommended dosage has not been established. (2.4 , 8.6 )
- Strong or Moderate CYP3A4 Inhibitors or Fluconazole: If coadministration with strong or moderate CYP3A4 inhibitors or fluconazole cannot be avoided, reduce the dose of KOSELUGO (see Tables 8 and 9) . (2.5 )
Recommended Dosage
The recommended dosage of KOSELUGO capsules ( see Table 1 ) and KOSELUGO oral granules ( see Table 2 ) for adult and pediatric patients 1 year of age and older, based on body surface area, is 25 mg/m 2 orally twice daily, until disease progression or unacceptable toxicity [see Dosage and Administration (2.2)].
Body Surface Area The recommended dosage of KOSELUGO capsules for patients with a BSA less than 0.55 m 2 has not been established. | KOSELUGO Capsules |
0.55 – 0.69 m 2 | 20 mg in the morning and 10 mg in the evening |
0.70 – 0.89 m 2 | 20 mg twice daily |
0.90 – 1.09 m 2 | 25 mg twice daily |
1.10 – 1.29 m 2 | 30 mg twice daily |
1.30 – 1.49 m 2 | 35 mg twice daily |
1.50 – 1.69 m 2 | 40 mg twice daily |
1.70 – 1.89 m 2 | 45 mg twice daily |
≥ 1.90 m 2 | 50 mg twice daily |
Body Surface Area The recommended dosage of KOSELUGO oral granules for patients with a BSA less than 0.40 m 2 has not been established. | KOSELUGO Oral Granules |
0.40 – 0.59 m 2 | 12.5 mg twice daily |
0.60 – 0.69 m 2 | 15 mg twice daily |
0.70 – 0.89 m 2 | 20 mg twice daily |
0.90 – 1.09 m 2 | 25 mg twice daily |
1.10 – 1.29 m 2 | 30 mg twice daily |
1.30 – 1.49 m 2 | 35 mg twice daily |
1.50 – 1.69 m 2 | 40 mg twice daily |
1.70 – 1.89 m 2 | 45 mg twice daily |
≥ 1.90 m 2 | 50 mg twice daily |
Administration
KOSELUGO is available in two dosage forms: KOSELUGO capsules and KOSELUGO oral granules. Prescribe KOSELUGO oral granules for patients who have difficulty swallowing whole capsules.
KOSELUGO Capsules
- Administer KOSELUGO capsules to patients who can swallow a whole capsule.
- Swallow KOSELUGO capsules whole. Do not open, chew or crush KOSELUGO capsules.
- KOSELUGO capsules may be administered with or without food.
KOSELUGO Oral Granules
Administer KOSELUGO oral granules to patients who have difficulty swallowing a whole capsule.
Sprinkle KOSELUGO oral granules on or mix with a small amount (about 1 to 3 teaspoons) of smooth yogurt, or fruit puree containing the following fruits: apple, banana, pear, or strawberry and consume within 30 minutes of preparation. If not consumed within 30 minutes of preparation, discard and prepare a new dose. If a dose has been partially consumed within 30 minutes of preparation, discard the remainder of the dose and do not prepare a new dose, aim to complete dosing within 30 minutes next time.
The KOSELUGO oral granules should be free-flowing. Do NOT use if the oral granules are clumped or stuck inside the capsule shell. Instruct the patient or caregiver to contact their pharmacy if this happens.
Discard the empty capsule shells after use.
Do NOT swallow, chew, or dissolve the capsule shells of KOSELUGO oral granules.
Do NOT chew or crush the KOSELUGO oral granules. Do NOT add oral granules to liquids.
Do NOT mix KOSELUGO oral granules in grapefruit or any juice, fruit puree or jam containing Seville orange.
Missed Dose
If a dose of KOSELUGO capsules or KOSELUGO oral granules is missed, make up that dose unless the next dose is due within 6 hours.
Vomiting
If vomiting occurs after taking a dose of KOSELUGO capsules or KOSELUGO oral granules, do not take an additional dose. Take the next dose at the regular scheduled time.
Dosage Modifications for Adverse Reactions
The recommended dose reductions for adverse reactions for KOSELUGO capsules and KOSELUGO oral granules are provided in Tables 3 and 4, respectively.
| Body Surface Area | First Dose Reduction (mg/dose) | Second Dose Reduction (mg/dose) | ||
|---|---|---|---|---|
| Morning | Evening | Morning | Evening | |
0.55 – 0.69 m 2 | 10 | 10 | 10 mg once daily | |
0.70 – 0.89 m 2 | 20 | 10 | 10 | 10 |
0.90 – 1.09 m 2 | 25 | 10 | 10 | 10 |
1.10 – 1.29 m 2 | 25 | 20 | 20 | 10 |
1.30 – 1.49 m 2 | 25 | 25 | 25 | 10 |
1.50 – 1.69 m 2 | 30 | 30 | 25 | 20 |
1.70 – 1.89 m 2 | 35 | 30 | 25 | 20 |
≥ 1.90 m 2 | 35 | 35 | 25 | 25 |
Permanently discontinue KOSELUGO capsules in patients unable to tolerate two dose reductions. | ||||
| Body Surface Area | First Dose Reduction (mg/dose) | Second Dose Reduction (mg/dose) | ||
|---|---|---|---|---|
| Morning | Evening | Morning | Evening | |
0.40 – 0.59 m 2 | 10 | 10 | 7.5 | 7.5 |
0.60 – 0.69 m 2 | 12.5 | 12.5 | 10 | 10 |
0.70 – 0.89 m 2 | 15 | 15 | 12.5 | 12.5 |
0.90 – 1.09 m 2 | 20 | 20 | 15 | 15 |
1.10 – 1.29 m 2 | 22.5 | 22.5 | 15 | 15 |
1.30 – 1.49 m 2 | 25 | 25 | 25 | 10 |
1.50 – 1.69 m 2 | 30 | 30 | 25 | 20 |
1.70 – 1.89 m 2 | 35 | 30 | 25 | 20 |
≥ 1.90 m 2 | 35 | 35 | 25 | 25 |
Permanently discontinue KOSELUGO oral granules in patients unable to tolerate two dose reductions. | ||||
The recommended dosage modifications of KOSELUGO capsules and KOSELUGO oral granules for adverse reactions are provided in Table 5.
| Severity of Adverse Reaction | Recommended Dosage Modifications for KOSELUGO capsules and KOSELUGO oral granules |
|---|---|
Left Ventricular Dysfunction [see Warnings and Precautions (5.1) ] | |
| Withhold until resolution. Resume at reduced dose. |
| Permanently discontinue. |
Ocular Toxicity [see Warnings and Precautions (5.2) ] | |
| Withhold until resolution. Resume at reduced dose. |
| Permanently discontinue. |
Gastrointestinal Toxicity [see Warnings and Precautions (5.3) ] | |
| Withhold until improved to Grade 0 or 1. Resume at same dose. Permanently discontinue if no improvement within 3 days. |
| Permanently discontinue. |
| Permanently discontinue. |
Skin Toxicity [see Warnings and Precautions (5.4) ] | |
| Withhold until improvement. Resume at reduced dose. |
Increased Creatine Phosphokinase (CPK) [see Warnings and Precautions (5.5) ] | |
| Withhold until improved to Grade 0 or 1. Resume at reduced dose. Permanently discontinue if no improvement within 3 weeks. |
| Permanently discontinue. |
Other Adverse Reactions [see Adverse Reactions (6.1)] | |
| Withhold KOSELUGO until improved to Grade 0 or 1. Resume at reduced dose. |
| Withhold KOSELUGO until improved to Grade 0 or 1. Resume at reduced dose. Consider discontinuation. |
• Per National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
Recommended Dosage in Patients with Hepatic Impairment
Severe Hepatic Impairment
The recommended dosage of KOSELUGO for use in patients with severe hepatic impairment (Child-Pugh C) has not been established [see Use in Specific Populations (8.6) ] .
Moderate Hepatic Impairment
The recommended dosage of KOSELUGO capsules (see Table 6) and KOSELUGO oral granules (see Table 7) for pediatric patients 1 year of age or older with moderate hepatic impairment (Child-Pugh B) is based on body surface area; 20 mg/m 2 orally twice daily, until disease progression or unacceptable toxicity [see Dosage and Administration (2.2) ].
| Body Surface Area | Moderate Hepatic Impairment (Child-Pugh B) (mg/dose) | |
|---|---|---|
| Morning | Evening | |
0.55 – 0.69 m 2 | 10 | 10 |
0.70 – 0.89 m 2 | 20 | 10 |
0.90 – 1.09 m 2 | 20 | 20 |
1.10 – 1.29 m 2 | 25 | 25 |
1.30 – 1.49 m 2 | 30 | 25 |
1.50 – 1.69 m 2 | 35 | 30 |
1.70 – 1.89 m 2 | 35 | 35 |
≥ 1.90 m 2 | 40 | 40 |
| Body Surface Area | Moderate Hepatic Impairment (Child‑Pugh B) (mg/dose) | |
|---|---|---|
| Morning | Evening | |
0.40 – 0.59 m 2 | 10 | 10 |
0.60 – 0.69 m 2 | 12.5 | 12.5 |
0.70 – 0.89 m 2 | 15 | 15 |
0.90 – 1.09 m 2 | 20 | 20 |
1.10 – 1.29 m 2 | 25 | 25 |
1.30 – 1.49 m 2 | 30 | 25 |
1.50 – 1.69 m 2 | 35 | 30 |
1.70 – 1.89 m 2 | 35 | 35 |
≥ 1.90 m 2 | 40 | 40 |
Dosage Modifications for Drug Interactions
Strong or Moderate CYP3A4 Inhibitors or Fluconazole
Avoid coadministration of strong or moderate CYP3A4 inhibitors or fluconazole with KOSELUGO. If coadministration with strong or moderate CYP3A4 inhibitors or fluconazole cannot be avoided, reduce the KOSELUGO dosage as recommended in Table 8 (KOSELUGO capsules) and Table 9 (KOSELUGO oral granules). After discontinuation of the strong or moderate CYP3A4 inhibitor or fluconazole for 3-elimination half-lives, resume the KOSELUGO dose that was taken prior to initiating the inhibitor or fluconazole [see Drug Interactions (7.1) ] .
| Body Surface Area | If the current dosage is 25 mg/m 2 twice daily, reduce to 20 mg/m 2 twice daily (mg/dose) | If the current dosage is 20 mg/m 2 twice daily, reduce to 15 mg/m 2 twice daily (mg/dose) | ||
|---|---|---|---|---|
| Morning | Evening | Morning | Evening | |
0.55 – 0.69 m 2 | 10 | 10 | 10 mg once daily | |
0.70 – 0.89 m 2 | 20 | 10 | 10 | 10 |
0.90 – 1.09 m 2 | 20 | 20 | 20 | 10 |
1.10 – 1.29 m 2 | 25 | 25 | 25 | 10 |
1.30 – 1.49 m 2 | 30 | 25 | 25 | 20 |
1.50 – 1.69 m 2 | 35 | 30 | 25 | 25 |
1.70 – 1.89 m 2 | 35 | 35 | 30 | 25 |
≥ 1.90 m 2 | 40 | 40 | 30 | 30 |
| Body Surface Area | If the current dosage is 25 mg/m 2 twice daily, reduce to 20 mg/m 2 twice daily (mg/dose) | If the current dosage is 20 mg/m 2 twice daily, reduce to 15 mg/m 2 twice daily (mg/dose) | ||
|---|---|---|---|---|
| Morning | Evening | Morning | Evening | |
0.40 – 0.59 m 2 | 10 | 10 | 7.5 | 7.5 |
0.60 – 0.69 m 2 | 12.5 | 12.5 | 10 | 7.5 |
0.70 – 0.89 m 2 | 15 | 15 | 10 | 10 |
0.90 – 1.09 m 2 | 20 | 20 | 15 | 15 |
1.10 – 1.29 m 2 | 25 | 25 | 25 | 10 |
1.30 – 1.49 m 2 | 30 | 25 | 25 | 20 |
1.50 – 1.69 m 2 | 35 | 30 | 25 | 25 |
1.70 – 1.89 m 2 | 35 | 35 | 30 | 25 |
≥ 1.90 m 2 | 40 | 40 | 30 | 30 |

By using PrescriberAI, you agree to the AI Terms of Use.
Koselugo prescribing information
INDICATIONS AND USAGE
KOSELUGO is indicated for the treatment of adult and pediatric patients 1 year of age and older with neurofibromatosis type 1 (NF1) who have symptomatic, inoperable plexiform neurofibromas (PN) [see Dosage and Administration (2) ].
DOSAGE AND ADMINISTRATION
- KOSELUGO capsules: The recommended dosage is 25 mg/m 2 , swallowed whole, taken orally twice daily with or without food (see Table 1) . (2.1 , 2.2 )
- KOSELUGO oral granules: The recommended dosage is equivalent to 25 mg/m 2 , sprinkled onto or mixed with soft food and taken orally twice daily (see Table 2). (2.1 , 2.2 )
- Moderate hepatic impairment (Child-Pugh B): The recommended dosage is 20 mg/m 2 orally twice daily (see Tables 6 and 7) . (2.2 , 2.4 )
- Severe hepatic impairment (Child-Pugh C): The recommended dosage has not been established. (2.4 , 8.6 )
- Strong or Moderate CYP3A4 Inhibitors or Fluconazole: If coadministration with strong or moderate CYP3A4 inhibitors or fluconazole cannot be avoided, reduce the dose of KOSELUGO (see Tables 8 and 9) . (2.5 )
Recommended Dosage
The recommended dosage of KOSELUGO capsules ( see Table 1 ) and KOSELUGO oral granules ( see Table 2 ) for adult and pediatric patients 1 year of age and older, based on body surface area, is 25 mg/m 2 orally twice daily, until disease progression or unacceptable toxicity [see Dosage and Administration (2.2)].
Body Surface Area The recommended dosage of KOSELUGO capsules for patients with a BSA less than 0.55 m 2 has not been established. | KOSELUGO Capsules |
0.55 – 0.69 m 2 | 20 mg in the morning and 10 mg in the evening |
0.70 – 0.89 m 2 | 20 mg twice daily |
0.90 – 1.09 m 2 | 25 mg twice daily |
1.10 – 1.29 m 2 | 30 mg twice daily |
1.30 – 1.49 m 2 | 35 mg twice daily |
1.50 – 1.69 m 2 | 40 mg twice daily |
1.70 – 1.89 m 2 | 45 mg twice daily |
≥ 1.90 m 2 | 50 mg twice daily |
Body Surface Area The recommended dosage of KOSELUGO oral granules for patients with a BSA less than 0.40 m 2 has not been established. | KOSELUGO Oral Granules |
0.40 – 0.59 m 2 | 12.5 mg twice daily |
0.60 – 0.69 m 2 | 15 mg twice daily |
0.70 – 0.89 m 2 | 20 mg twice daily |
0.90 – 1.09 m 2 | 25 mg twice daily |
1.10 – 1.29 m 2 | 30 mg twice daily |
1.30 – 1.49 m 2 | 35 mg twice daily |
1.50 – 1.69 m 2 | 40 mg twice daily |
1.70 – 1.89 m 2 | 45 mg twice daily |
≥ 1.90 m 2 | 50 mg twice daily |
Administration
KOSELUGO is available in two dosage forms: KOSELUGO capsules and KOSELUGO oral granules. Prescribe KOSELUGO oral granules for patients who have difficulty swallowing whole capsules.
KOSELUGO Capsules
- Administer KOSELUGO capsules to patients who can swallow a whole capsule.
- Swallow KOSELUGO capsules whole. Do not open, chew or crush KOSELUGO capsules.
- KOSELUGO capsules may be administered with or without food.
KOSELUGO Oral Granules
Administer KOSELUGO oral granules to patients who have difficulty swallowing a whole capsule.
Sprinkle KOSELUGO oral granules on or mix with a small amount (about 1 to 3 teaspoons) of smooth yogurt, or fruit puree containing the following fruits: apple, banana, pear, or strawberry and consume within 30 minutes of preparation. If not consumed within 30 minutes of preparation, discard and prepare a new dose. If a dose has been partially consumed within 30 minutes of preparation, discard the remainder of the dose and do not prepare a new dose, aim to complete dosing within 30 minutes next time.
The KOSELUGO oral granules should be free-flowing. Do NOT use if the oral granules are clumped or stuck inside the capsule shell. Instruct the patient or caregiver to contact their pharmacy if this happens.
Discard the empty capsule shells after use.
Do NOT swallow, chew, or dissolve the capsule shells of KOSELUGO oral granules.
Do NOT chew or crush the KOSELUGO oral granules. Do NOT add oral granules to liquids.
Do NOT mix KOSELUGO oral granules in grapefruit or any juice, fruit puree or jam containing Seville orange.
Missed Dose
If a dose of KOSELUGO capsules or KOSELUGO oral granules is missed, make up that dose unless the next dose is due within 6 hours.
Vomiting
If vomiting occurs after taking a dose of KOSELUGO capsules or KOSELUGO oral granules, do not take an additional dose. Take the next dose at the regular scheduled time.
Dosage Modifications for Adverse Reactions
The recommended dose reductions for adverse reactions for KOSELUGO capsules and KOSELUGO oral granules are provided in Tables 3 and 4, respectively.
| Body Surface Area | First Dose Reduction (mg/dose) | Second Dose Reduction (mg/dose) | ||
|---|---|---|---|---|
| Morning | Evening | Morning | Evening | |
0.55 – 0.69 m 2 | 10 | 10 | 10 mg once daily | |
0.70 – 0.89 m 2 | 20 | 10 | 10 | 10 |
0.90 – 1.09 m 2 | 25 | 10 | 10 | 10 |
1.10 – 1.29 m 2 | 25 | 20 | 20 | 10 |
1.30 – 1.49 m 2 | 25 | 25 | 25 | 10 |
1.50 – 1.69 m 2 | 30 | 30 | 25 | 20 |
1.70 – 1.89 m 2 | 35 | 30 | 25 | 20 |
≥ 1.90 m 2 | 35 | 35 | 25 | 25 |
Permanently discontinue KOSELUGO capsules in patients unable to tolerate two dose reductions. | ||||
| Body Surface Area | First Dose Reduction (mg/dose) | Second Dose Reduction (mg/dose) | ||
|---|---|---|---|---|
| Morning | Evening | Morning | Evening | |
0.40 – 0.59 m 2 | 10 | 10 | 7.5 | 7.5 |
0.60 – 0.69 m 2 | 12.5 | 12.5 | 10 | 10 |
0.70 – 0.89 m 2 | 15 | 15 | 12.5 | 12.5 |
0.90 – 1.09 m 2 | 20 | 20 | 15 | 15 |
1.10 – 1.29 m 2 | 22.5 | 22.5 | 15 | 15 |
1.30 – 1.49 m 2 | 25 | 25 | 25 | 10 |
1.50 – 1.69 m 2 | 30 | 30 | 25 | 20 |
1.70 – 1.89 m 2 | 35 | 30 | 25 | 20 |
≥ 1.90 m 2 | 35 | 35 | 25 | 25 |
Permanently discontinue KOSELUGO oral granules in patients unable to tolerate two dose reductions. | ||||
The recommended dosage modifications of KOSELUGO capsules and KOSELUGO oral granules for adverse reactions are provided in Table 5.
| Severity of Adverse Reaction | Recommended Dosage Modifications for KOSELUGO capsules and KOSELUGO oral granules |
|---|---|
Left Ventricular Dysfunction [see Warnings and Precautions (5.1) ] | |
| Withhold until resolution. Resume at reduced dose. |
| Permanently discontinue. |
Ocular Toxicity [see Warnings and Precautions (5.2) ] | |
| Withhold until resolution. Resume at reduced dose. |
| Permanently discontinue. |
Gastrointestinal Toxicity [see Warnings and Precautions (5.3) ] | |
| Withhold until improved to Grade 0 or 1. Resume at same dose. Permanently discontinue if no improvement within 3 days. |
| Permanently discontinue. |
| Permanently discontinue. |
Skin Toxicity [see Warnings and Precautions (5.4) ] | |
| Withhold until improvement. Resume at reduced dose. |
Increased Creatine Phosphokinase (CPK) [see Warnings and Precautions (5.5) ] | |
| Withhold until improved to Grade 0 or 1. Resume at reduced dose. Permanently discontinue if no improvement within 3 weeks. |
| Permanently discontinue. |
Other Adverse Reactions [see Adverse Reactions (6.1)] | |
| Withhold KOSELUGO until improved to Grade 0 or 1. Resume at reduced dose. |
| Withhold KOSELUGO until improved to Grade 0 or 1. Resume at reduced dose. Consider discontinuation. |
• Per National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
Recommended Dosage in Patients with Hepatic Impairment
Severe Hepatic Impairment
The recommended dosage of KOSELUGO for use in patients with severe hepatic impairment (Child-Pugh C) has not been established [see Use in Specific Populations (8.6) ] .
Moderate Hepatic Impairment
The recommended dosage of KOSELUGO capsules (see Table 6) and KOSELUGO oral granules (see Table 7) for pediatric patients 1 year of age or older with moderate hepatic impairment (Child-Pugh B) is based on body surface area; 20 mg/m 2 orally twice daily, until disease progression or unacceptable toxicity [see Dosage and Administration (2.2) ].
| Body Surface Area | Moderate Hepatic Impairment (Child-Pugh B) (mg/dose) | |
|---|---|---|
| Morning | Evening | |
0.55 – 0.69 m 2 | 10 | 10 |
0.70 – 0.89 m 2 | 20 | 10 |
0.90 – 1.09 m 2 | 20 | 20 |
1.10 – 1.29 m 2 | 25 | 25 |
1.30 – 1.49 m 2 | 30 | 25 |
1.50 – 1.69 m 2 | 35 | 30 |
1.70 – 1.89 m 2 | 35 | 35 |
≥ 1.90 m 2 | 40 | 40 |
| Body Surface Area | Moderate Hepatic Impairment (Child‑Pugh B) (mg/dose) | |
|---|---|---|
| Morning | Evening | |
0.40 – 0.59 m 2 | 10 | 10 |
0.60 – 0.69 m 2 | 12.5 | 12.5 |
0.70 – 0.89 m 2 | 15 | 15 |
0.90 – 1.09 m 2 | 20 | 20 |
1.10 – 1.29 m 2 | 25 | 25 |
1.30 – 1.49 m 2 | 30 | 25 |
1.50 – 1.69 m 2 | 35 | 30 |
1.70 – 1.89 m 2 | 35 | 35 |
≥ 1.90 m 2 | 40 | 40 |
Dosage Modifications for Drug Interactions
Strong or Moderate CYP3A4 Inhibitors or Fluconazole
Avoid coadministration of strong or moderate CYP3A4 inhibitors or fluconazole with KOSELUGO. If coadministration with strong or moderate CYP3A4 inhibitors or fluconazole cannot be avoided, reduce the KOSELUGO dosage as recommended in Table 8 (KOSELUGO capsules) and Table 9 (KOSELUGO oral granules). After discontinuation of the strong or moderate CYP3A4 inhibitor or fluconazole for 3-elimination half-lives, resume the KOSELUGO dose that was taken prior to initiating the inhibitor or fluconazole [see Drug Interactions (7.1) ] .
| Body Surface Area | If the current dosage is 25 mg/m 2 twice daily, reduce to 20 mg/m 2 twice daily (mg/dose) | If the current dosage is 20 mg/m 2 twice daily, reduce to 15 mg/m 2 twice daily (mg/dose) | ||
|---|---|---|---|---|
| Morning | Evening | Morning | Evening | |
0.55 – 0.69 m 2 | 10 | 10 | 10 mg once daily | |
0.70 – 0.89 m 2 | 20 | 10 | 10 | 10 |
0.90 – 1.09 m 2 | 20 | 20 | 20 | 10 |
1.10 – 1.29 m 2 | 25 | 25 | 25 | 10 |
1.30 – 1.49 m 2 | 30 | 25 | 25 | 20 |
1.50 – 1.69 m 2 | 35 | 30 | 25 | 25 |
1.70 – 1.89 m 2 | 35 | 35 | 30 | 25 |
≥ 1.90 m 2 | 40 | 40 | 30 | 30 |
| Body Surface Area | If the current dosage is 25 mg/m 2 twice daily, reduce to 20 mg/m 2 twice daily (mg/dose) | If the current dosage is 20 mg/m 2 twice daily, reduce to 15 mg/m 2 twice daily (mg/dose) | ||
|---|---|---|---|---|
| Morning | Evening | Morning | Evening | |
0.40 – 0.59 m 2 | 10 | 10 | 7.5 | 7.5 |
0.60 – 0.69 m 2 | 12.5 | 12.5 | 10 | 7.5 |
0.70 – 0.89 m 2 | 15 | 15 | 10 | 10 |
0.90 – 1.09 m 2 | 20 | 20 | 15 | 15 |
1.10 – 1.29 m 2 | 25 | 25 | 25 | 10 |
1.30 – 1.49 m 2 | 30 | 25 | 25 | 20 |
1.50 – 1.69 m 2 | 35 | 30 | 25 | 25 |
1.70 – 1.89 m 2 | 35 | 35 | 30 | 25 |
≥ 1.90 m 2 | 40 | 40 | 30 | 30 |
DOSAGE FORMS AND STRENGTHS
Capsules:
- 10 mg selumetinib: white to off-white, opaque, hard capsule sealed with a clear band and marked with “SEL 10” in black ink.
- 25 mg selumetinib: blue, opaque, hard capsule sealed with a clear band and marked with “SEL 25” in black ink.
Oral Granules:
- 5 mg selumetinib: off-white to light-yellow free-flowing oral granules contained within capsules. The capsules have a yellow cap and white body. The cap is printed with “sel 5” in black ink, and body is printed with a sprinkle capsule image indicating opening.
- 7.5 mg selumetinib: off-white to light-yellow free-flowing oral granules contained within capsules. The capsules have a pink cap and white body. The cap is printed with “sel 7.5” in black ink, and body is printed with a sprinkle capsule image indicating opening.
USE IN SPECIFIC POPULATIONS
- Lactation: Advise not to breastfeed. (8.2)
Pregnancy
Risk Summary
Based on findings from animal studies and its mechanism of action [see Clinical Pharmacology (12.1) ] , KOSELUGO can cause fetal harm when administered to a pregnant woman. There are no available data on the use of KOSELUGO in pregnant women to evaluate drug-associated risk. In animal reproduction studies, administration of selumetinib to mice during organogenesis caused reduced fetal weight, adverse structural defects, and effects on embryofetal survival at exposures approximately > 5 times the human exposure at the clinical dose of 25 mg/m 2 twice daily ( see Data ). Advise pregnant women of the potential risk to the fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Human Data
In KOMET, a first trimester spontaneous abortion was reported in a patient receiving KOSELUGO.
Animal Data
In embryo-fetal development studies in mice at doses > 2.5 mg/kg twice daily (~5-times the human exposure based on area under the curve [AUC] at the clinical dose of 25 mg/m 2 twice daily), selumetinib caused increases in post-implantation loss, a reduction in mean fetal and litter weights, and an increased occurrence of open eye and cleft palate, but did not induce significant maternal toxicity.
Administration of selumetinib to pregnant mice from gestation Day 6 through lactation Day 20 resulted in reduced pup body weights and fewer pups met the pupil constriction criterion on day 21 post-partum. The incidence of malformations (e.g., prematurely open eye(s) and cleft palate) was increased even at the lowest dose of 0.5 mg/kg twice daily (maternal maximal concentration [C max ] of ~0.6 times the human C max at the clinical dose of 25 mg/m 2 twice daily).
Lactation
Risk Summary
There are no data on the presence of selumetinib or its active metabolite in human milk or their effects on the breastfed child or milk production. Selumetinib and its active metabolite were present in the milk of lactating mice ( see Data ). Due to the potential for adverse reactions in a breastfed child, advise women not to breastfeed during treatment with KOSELUGO and for 1 week after the last dose.
Data
Animal Data
Selumetinib and its active metabolite were present in milk from mice dosed with selumetinib throughout gestation and lactation, with a mean plasma/milk ratio of 1.5 in lactating dams dosed at 5 mg/kg twice daily. Administration of selumetinib to dams during gestation and early lactation was associated with adverse events in pups, including reduced growth rates and incidence of malformations [see Use in Specific Populations (8.1) ].
Females and Males of Reproductive Potential
KOSELUGO can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1) ] .
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating KOSELUGO [see Use in Specific Populations (8.1) ] .
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment and for 1 week after the last dose.
Males
Advise male patients with female partners of reproductive potential to use effective contraception during treatment with KOSELUGO and for 1 week after the last dose.
Pediatric Use
The safety and effectiveness have been established in pediatric patients 1 year of age and older with NF1 who have inoperable PN and the information on this use is discussed throughout the labeling. The safety and effectiveness of KOSELUGO have not been established in pediatric patients younger than 1 year of age.
Animal Toxicity Data
In 3-month general toxicology studies, male rats receiving selumetinib at doses ≥ 10 mg/kg daily (~60-times the human exposure based on AUC at the clinical dose of 25 mg/m 2 twice daily) showed growth plate dysplasia.
Geriatric Use
Clinical studies of KOSELUGO did not include a sufficient number of patients aged 65 and over to determine whether they respond differently from younger patients.
Hepatic Impairment
Selumetinib exposures increased in patients with moderate or severe hepatic impairment [see Clinical Pharmacology (12.3) ] . Reduce the dose of KOSELUGO for patients with moderate hepatic impairment (Child-Pugh B). A recommended dosage of KOSELUGO for use in patients with severe hepatic impairment (Child-Pugh C) has not been established [see Dosage and Administration (2.4) ].
CONTRAINDICATIONS
None.
WARNINGS AND PRECAUTIONS
- Left Ventricular Dysfunction : Assess ejection fraction prior to initiating treatment, every 3 months during the first year, then every 6 months thereafter and as clinically indicated. Withhold, reduce the dose, or permanently discontinue KOSELUGO based on severity of adverse reaction. (2.3 , 5.1 )
- Ocular Toxicity : Conduct ophthalmic assessments prior to initiating KOSELUGO, at regular intervals during treatment and for new or worsening visual changes. Permanently discontinue KOSELUGO for retinal vein occlusion (RVO). Withhold KOSELUGO for retinal pigment epithelial detachment (RPED), monitor with optical coherence tomography assessments until resolution, and resume at reduced dose. (2.3 , 5.2 )
- Gastrointestinal Toxicity : Advise patients to start an anti-diarrheal agent immediately after the first episode of loose stool and to increase fluid intake. Withhold, reduce the dose, or permanently discontinue KOSELUGO based on severity of adverse reaction. (2.3 , 5.3 )
- Skin Toxicity : Monitor for severe skin rashes. Withhold, reduce the dose, or permanently discontinue KOSELUGO based on severity of adverse reaction. (2.3 , 5.4 )
- Increased Creatine Phosphokinase (CPK) : Increased CPK and rhabdomyolysis can occur. Obtain serum CPK prior to initiating KOSELUGO, periodically during treatment, and as clinically indicated. If increased CPK occurs, evaluate for rhabdomyolysis or other causes. Withhold, reduce the dose, or permanently discontinue KOSELUGO based on severity of adverse reaction. (2.3 , 5.5 )
- Increased Vitamin E Levels and Increased Risk of Bleeding (KOSELUGO Capsules) : KOSELUGO capsules contain vitamin E and daily intake of vitamin E that exceeds the recommended or safe limits may increase the risk of bleeding. An increased risk of bleeding may occur in patients co-administered vitamin-K antagonists or anti-platelet agents. KOSELUGO oral granules do not contain vitamin E. (5.6 )
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise patients of reproductive potential of the potential risk to a fetus and to use effective contraception (5.7 , 8.1 , 8.3 ).
Left Ventricular Dysfunction
KOSELUGO can cause cardiomyopathy, defined as a decrease in left ventricular ejection fraction (LVEF) ≥ 10% below baseline. KOSELUGO has not been studied in patients with a history of clinically significant cardiac disease or LVEF less than 55% prior to treatment.
Pediatric patients
In the NF1 PN pediatric safety pool (N = 134) [see Adverse Reactions (6.1) ], Grade 2 LVEF decrease [Grade 2 LVEF decrease (40% to 50%; 10 to 19% drop from baseline)], based on reported adverse reactions, occurred in 17% of evaluable patients. Decreased LVEF of ≥ 20% occurred in 0.7% of patients and resulted in dose interruption and dose reduction. Decreased LVEF resolved in 75% of these patients. The median time to first occurrence of LVEF decrease was approximately 12 months (median duration approximately 3 months).
Adult Patients
In the KOMET adult NF1 PN study (N = 71) [see Adverse Reactions (6.1) ], Grade 2 LVEF decrease [Grade 2 LVEF decrease (40% to 50%; 10 to 19% drop from baseline)], based on echocardiogram results, occurred in 14% of evaluable patients. Decreased LVEF resulted in dose interruption in 1.4% of patients. The median time to first occurrence of LVEF decrease was approximately 4 months (median duration approximately 4 months).
Assess ejection fraction by echocardiogram prior to initiating treatment, every 3 months during the first year of treatment, every 6 months thereafter, and as clinically indicated. Withhold, reduce dose, or permanently discontinue KOSELUGO based on severity of adverse reaction [see Dosage and Administration (2.3)]. In patients who interrupt KOSELUGO for decreased LVEF, obtain an echocardiogram or a cardiac MRI every 3 to 6 weeks until resolution. Upon resolution of decreased LVEF to greater than or equal to the institutional LLN, obtain an echocardiogram or a cardiac MRI every 2 to 3 months or as directed by the cardiologist.
Ocular Toxicity
KOSELUGO can cause ocular toxicity, including retinal vein occlusion (RVO), retinal pigment epithelial detachment (RPED), and blurred vision.
Pediatric Patients
In the NF1 PN pediatric safety pool (N = 134) [see Adverse Reactions (6.1) ], blurred vision, photophobia, cataracts, ocular hypertension, and retinal tear occurred in 13% of pediatric patients receiving KOSELUGO. Blurred vision resulted in dose interruption in 1.5% of patients. Ocular toxicity resolved in 76% of these patients. RPED occurred in the pediatric population during treatment with single agent KOSELUGO and resulted in permanent discontinuation.
Adult Patients
In the KOMET adult NF1 PN study (N = 71) [see Adverse Reactions (6.1) ], blurred vision and vitreous floaters occurred in 6% of patients receiving KOSELUGO. Serious ocular toxicities including RVO and RPED, occurred in an unapproved population of adult patients with multiple tumor types who received KOSELUGO as a single agent or in combination with other anti-cancer agents.
Conduct comprehensive ophthalmic assessments prior to initiating KOSELUGO, at regular intervals during treatment, and for new or worsening visual changes. Permanently discontinue KOSELUGO in patients with RVO. Withhold KOSELUGO in patients with RPED, follow up with optical coherence tomography assessments every 3 weeks until resolution, and resume KOSELUGO at a reduced dose. For other ocular toxicities, withhold, reduce dose, or permanently discontinue KOSELUGO based on severity of the adverse reaction [see Dosage and Administration (2.3) ].
Gastrointestinal Toxicity
KOSELUGO can cause gastrointestinal toxicities, including diarrhea and colitis.
Pediatric Patients
In the NF1 PN pediatric safety pool (N = 134) [see Adverse Reactions (6.1) ] , diarrhea occurred in 59% of patients who received KOSELUGO, including Grade 3 in 10% of patients. Diarrhea resulting in permanent discontinuation occurred in 0.7% of patients. Diarrhea resulting in dose interruption occurred in 10% of patients. The median time to first onset of diarrhea was approximately 2 months and the median duration was 5 days. Colitis occurred in an unapproved population of pediatric patients with multiple tumor types who received KOSELUGO as a single agent.
Adult Patients
In the KOMET adult NF1 PN study (N = 71) [see Adverse Reactions (6.1) ] , diarrhea occurred in 42% patients who received KOSELUGO. Diarrhea resulting in dose interruption occurred in 1.4% of patients. The median time to first onset of diarrhea was approximately 1 month and the median duration was 7 days. Serious gastrointestinal toxicities, including perforation, colitis, ileus, and intestinal obstruction, occurred in an unapproved population of adult patients with multiple tumor types who received KOSELUGO as a single agent or in combination with other anti-cancer agents.
Advise patients to start an anti-diarrheal agent (e.g., loperamide) immediately after the first episode of unformed, loose stool and to increase fluid intake during diarrhea episodes. Withhold, reduce dose, or permanently discontinue KOSELUGO based on severity of adverse reaction [see Dosage and Administration (2.3) ].
Skin Toxicity
KOSELUGO can cause severe rashes, including dermatitis acneiform.
Pediatric Patients
In the NF1 PN pediatric safety pool (N = 134) [see Adverse Reactions (6.1) ] , rash occurred in 68% of patients who received KOSELUGO. The most frequent rashes included dermatitis acneiform (47%) and maculopapular rash (31%). Pruritus (30%), alopecia (26%), and eczema (24%) occurred in patients who received KOSELUGO. Grade 3 rash occurred in 5% of patients. Rash resulted in dose interruption in 8% of patients and dose reduction in 3.7% of patients.
Adult Patients
In the KOMET adult NF1 PN study (N = 71) [see Adverse Reactions (6.1) ], rash occurred in 85% of patients who received KOSELUGO. The most frequent rash included dermatitis acneiform (66%). Alopecia (18%) and pruritus (10%) occurred in patients who received KOSELUGO. Grade 3 rash occurred in 4.2% of patients. Rash resulted in dose interruption in 2.8% of patients, dose reduction in 2.8% of patients, and permanent discontinuation in 2.8% of patients.
Other skin toxicities, including severe palmar-plantar erythrodysesthesia syndrome, occurred in an unapproved population of adult patients with multiple tumor types who received KOSELUGO as a single agent or in combination with other anti-cancer agents.
Monitor for severe skin rashes. Withhold, reduce dose, or permanently discontinue KOSELUGO based on severity of adverse reaction [see Dosage and Administration (2.3) ].
Increased Creatine Phosphokinase
KOSELUGO can cause increased creatine phosphokinase (CPK), myalgia, and rhabdomyolysis.
Pediatric Patients
In the NF1 PN pediatric safety pool (N = 134) [see Adverse Reactions (6.1) ] , increased creatine phosphokinase (CPK), based on laboratory data, occurred in 73% of patients who received KOSELUGO, including Grade 3 or 4 in 8% of patients. Increased CPK resulted in dose interruption and dose reduction in 4% of patients. Increased CPK concurrent with myalgia occurred in 5% of patients, including one patient who permanently discontinued KOSELUGO for myalgia.
Adults
In the KOMET adult NF1 PN study (N = 71) [see Adverse Reactions (6.1 )] , increased creatine phosphokinase (CPK), based on laboratory data, occurred in 70% of patients who received KOSELUGO, including Grade 3 or 4 in 7% of patients. Increased CPK resulted in dose interruption and dose reduction in 4.2% and 2.8% of patients, respectively. Increased CPK concurrent with myalgia occurred in 1.4% of patients. Rhabdomyolysis occurred in an unapproved adult population who received KOSELUGO as a single agent.
Obtain serum CPK prior to initiating KOSELUGO, periodically during treatment, and as clinically indicated. If increased CPK occurs, evaluate patients for rhabdomyolysis or other causes. Withhold, reduce dose, or permanently discontinue KOSELUGO based on severity of adverse reaction [see Dosage and Administration (2.3) ] .
Increased Levels of Vitamin E and Increased Risk of Bleeding (KOSELUGO Capsules)
KOSELUGO capsules can cause increased levels of vitamin E and increased risk of bleeding.
KOSELUGO capsules contain vitamin E (10 mg capsules contain 32 mg vitamin E as the excipient, D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS); while KOSELUGO 25 mg capsules contain 36 mg vitamin E as TPGS). Vitamin E can inhibit platelet aggregation and antagonize vitamin K-dependent clotting factors. Daily vitamin E intake that exceeds the recommended or safe limits may increase the risk of bleeding. Supplemental vitamin E is not recommended if daily vitamin E intake (including the amount of vitamin E in KOSELUGO and supplement) will exceed the recommended or safe limits.
An increased risk of bleeding in patients may occur in patients who are co-administered vitamin K antagonists or anti-platelet antagonists with KOSELUGO capsules. Monitor for bleeding in these patients. Increase international normalized ratio (INR) monitoring, as appropriate, in patients taking a vitamin K antagonist. Perform anticoagulant assessments, including INR or prothrombin time, more frequently and adjust the dose of vitamin K antagonists or anti-platelet agents as appropriate.
KOSELUGO oral granules do not contain vitamin E [see Drug Interactions (7.1) ].
Embryo-Fetal Toxicity
Based on findings from clinical trials, animal studies and its mechanism of action, KOSELUGO can cause fetal harm when administered to a pregnant woman. In KOMET, a first trimester spontaneous abortion was reported in a patient receiving KOSELUGO.
In animal reproduction studies, administration of selumetinib to mice during organogenesis caused reduced fetal weight, adverse structural defects, and effects on embryo fetal survival at approximate exposures > 5 times the human exposure at the clinical dose of 25 mg/m 2 twice daily. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with KOSELUGO and for 1 week after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with KOSELUGO and for 1 week after the last dose [see Use in Specific Populations (8.1 , 8.3) ] .
ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Left Ventricular Dysfunction [see Warnings and Precautions (5.1) ]
- Ocular Toxicity [see Warnings and Precautions (5.2) ]
- Gastrointestinal Toxicity [see Warnings and Precautions (5.3) ]
- Skin Toxicity [see Warnings and Precautions (5.4) ]
- Increased Creatine Phosphokinase [see Warnings and Precautions (5.5) ]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The NF1 PN pediatric safety pool described in the WARNINGS AND PRECAUTIONS reflects exposure to KOSELUGO at the recommended dosage in 134 pediatric patients in SPRINKLE (N = 36) (NCT05309668), SPRINT Phase I (N = 24) (NCT01362803), SPRINT Phase II Stratum 1 (N = 50) [see Clinical Studies (14.1) ] , and Phase I Food Effect Study (N = 24) (NCT05101148). Among pediatric patients, the duration of KOSELUGO exposure was 12 months or longer (80%), more than 2 years (44%), or more than 3 years (37%). The most common adverse reactions in pediatric patients (≥ 40%) are vomiting, diarrhea, increased creatine phosphokinase, dry skin, paronychia, nausea, dermatitis acneiform, and pyrexia.
In the KOMET adult NF1 PN study, 71 adult patients received KOSELUGO at the recommended dosage [see Clinical Studies (14.1) ] . Among adult patients, the duration of KOSELUGO exposure in the randomized period was 6 months or longer (92%), and 11 months or longer (66%). The most common adverse reactions in adult patients (≥ 40%) are rash (all), dermatitis acneiform, and diarrhea.
Neurofibromatosis Type 1 (NF1) with Inoperable Plexiform Neurofibromas (PN)
Pediatrics 2-18 years of Age (SPRINT Phase II Stratum 1)
The safety of KOSELUGO was evaluated in SPRINT Phase II Stratum 1 [see Clinical Studies (14.1) ] . Eligible patients were 2-18 years of age with neurofibromatosis type 1 (NF1) who had inoperable plexiform neurofibromas (PN) that was causing significant morbidity. Patients were excluded for abnormal LVEF, uncontrolled hypertension (blood pressure ≥ the 95th percentile for age, height, and sex), any current or past history of RVO or RPED, intraocular pressure > 21 mmHg (or upper limit of normal adjusted by age), uncontrolled glaucoma, and inability to swallow whole capsules. Patients received KOSELUGO 25 mg/m 2 orally twice daily (N = 50). Among these patients, 88% were exposed for 12 months or longer and 66% were exposed for greater than 2 years.
Serious adverse reactions occurred in 24% of patients who received KOSELUGO. Serious adverse reactions that occurred in 2 or more patients were anemia, hypoxia and diarrhea.
Permanent discontinuation due to an adverse reaction occurred in 12% of patients who received KOSELUGO. Adverse reactions resulting in permanent discontinuation of KOSELUGO included increased blood creatinine, increased weight, diarrhea, paronychia, malignant peripheral nerve sheath tumor, acute kidney injury, and skin ulcer.
Dosage interruptions and dose reductions due to adverse reactions occurred in 80% and 24% of patients who received KOSELUGO, respectively. Adverse reactions requiring a dosage interruption or reduction in ≥ 5% of patients were vomiting, paronychia, diarrhea, nausea, abdominal pain, rash, skin infection, influenza-like illness, pyrexia and weight gain.
The most common adverse reactions (≥ 40%) were vomiting, rash (all), abdominal pain, diarrhea, nausea, dry skin, fatigue, musculoskeletal pain, pyrexia, acneiform rash, stomatitis, headache, paronychia, and pruritus.
Table 10 presents the adverse reactions in SPRINT Phase II Stratum 1.
Adverse Reaction | KOSELUGO (N = 50) | |
All Grades (%) | Grade ≥ 3 (%) • | |
Gastrointestinal | ||
Vomiting | 82 | 6 |
Abdominal pain Abdominal pain includes abdominal pain; abdominal pain upper | 76 | 0 |
Diarrhea | 70 | 16 |
Nausea | 66 | 2 |
Stomatitis Stomatitis includes stomatitis; mouth ulceration | 50 | 0 |
Constipation | 34 | 0 |
Skin and Subcutaneous Tissue | ||
Rash (all) Rash (all) includes dermatitis acneiform; rash maculo-papular; erythema; rash pustular; rash; urticaria; exfoliative rash; rash pruritic; rash erythematous | 80 | 6 |
Dry skin | 60 | 0 |
Rash acneiform Rash (acneiform) includes dermatitis acneiform | 50 | 4 |
Paronychia Paronychia includes paronychia; nail infection | 48 | 6 |
Pruritus | 46 | 0 |
Dermatitis Dermatitis includes dermatitis; dermatitis atopic; dermatitis diaper; eczema; seborrheic dermatitis; skin irritation | 36 | 4 |
Hair changes Hair changes include alopecia; hair color change | 32 | 0 |
Musculoskeletal and Connective Tissue | ||
Musculoskeletal pain Musculoskeletal pain includes pain in extremity; back pain; neck pain; musculoskeletal pain | 58 | 0 |
General | ||
Fatigue Fatigue includes fatigue; malaise | 56 | 0 |
Pyrexia | 56 | 8 |
Edema Edema includes peripheral swelling; edema; localized edema | 20 | 0 |
Nervous System | ||
Headache | 48 | 2 |
Respiratory, Thoracic and Mediastinal | ||
Epistaxis | 28 | 0 |
Renal and Urinary System | ||
Hematuria | 22 | 2 |
Proteinuria | 22 | 0 |
Metabolism and Nutrition | ||
Decreased appetite | 22 | 0 |
Cardiac System | ||
Decreased ejection fraction | 22 | 0 |
Sinus tachycardia | 20 | 0 |
Infections | ||
Skin infection Skin infection includes skin infection; abscess; cellulitis; impetigo; staphylococcal skin infection | 20 | 2 |
• All events were Grade 3. | ||
Clinically relevant adverse reactions that occurred < 20% of patients include:
- Eye: visual impairment.
- Gastrointestinal Disorders: dry mouth.
- General Disorders: facial edema, including periorbital edema and face edema.
- Metabolism and Nutrition: increased weigh.t
- Renal and Urinary System: acute kidney injury.
- Respiratory, Thoracic & Mediastinal: dyspnea, including exertional dyspnea and dyspnea at rest.
- Vascular: hypertension.
Table 11 presents the laboratory abnormalities in SPRINT Phase II Stratum 1.
Laboratory Abnormality | KOSELUGO | |
All Grades (%) The denominator used to calculate the rate varied from 39 to 49 based on the number of patients with a baseline value and at least one post-treatment value. | Grade ≥ 3 (%) | |
Chemistry | ||
Increased creatine phosphokinase (CPK) | 79 | 7 Includes one Grade 4 increased CPK and one Grade 4 increased potassium. |
Decreased albumin | 51 | 0 |
Increased aspartate aminotransferase (AST) | 41 | 2 |
Increased alanine aminotransferase (ALT) | 35 | 4 |
Increased lipase | 32 | 5 |
Increased potassium | 27 | 4 |
Decreased potassium | 18 | 2 § |
Increased alkaline phosphatase | 18 | 0 |
Increased amylase | 18 | 0 |
Increased sodium | 18 | 0 |
Decreased sodium | 16 | 0 |
Hematology | ||
Decreased hemoglobin | 41 | 4 |
Decreased neutrophils | 33 | 4 |
Decreased lymphocytes | 20 | 2 |
Adults ≥ 18 years of Age (KOMET)
The safety of KOSELUGO was evaluated in KOMET [see Clinical Studies (14.1) ] . Eligible patients were 18 years of age or older with NF1 who had symptomatic, inoperable PN. Patients were excluded for abnormal LVEF, uncontrolled hypertension, any current or past history of RVO or RPED/CSR, intraocular pressure > 21 mmHg (or upper limit of normal adjusted by age), uncontrolled glaucoma, and inability to swallow whole capsules. Among the patients (N = 137) who have received KOSELUGO, the median duration of KOSELUGO treatment was 11 months with a range of 10 days to 31 months.
Serious adverse reactions occurred in 14% of patients who received KOSELUGO. Serious adverse reactions occurring in more than one patient included cellulitis (2.8%).
Permanent discontinuation due to an adverse reaction occurred in 13% of patients who received KOSELUGO. Adverse reactions resulting in permanent discontinuation of KOSELUGO included dermatitis acneiform, cellulitis, nausea, wound, neurofibrosarcoma, neurofibrosarcoma recurrent, psychiatric decompensation, ulcerative keratitis, and nail disorder.
Dosage interruptions and dose reductions due to adverse reactions occurred in 27% and 14% of patients who received KOSELUGO, respectively. Adverse reactions requiring a dosage reduction in 2 or more patients were paronychia, increased CPK, increased ALT, increased AST, rash, and alopecia. Adverse reactions requiring a dosage interruption in 2 or more patients were increased CPK, rash, headache, abdominal pain, nausea, and COVID‑19.
The most common adverse reactions (≥ 40%) were rash (all), rash (acneiform), and diarrhea.
Table 12 presents the adverse reactions in the KOMET study. The 12 cycle (48 weeks) randomization period for KOSELUGO versus placebo was followed by a single arm treatment period where all patients received KOSELUGO (placebo patients crossed over to KOSELUGO at end of the randomized period). No new adverse reactions were identified during the open-label period.
| Adverse Reactions | Randomized to KOSELUGO • (N = 71) | Randomized to Placebo • (N = 74) | ||
|---|---|---|---|---|
| All Grades (%) | Grades ≥ 3 (%) | All Grades (%) | Grades ≥ 3 (%) | |
| ||||
| 85 | 4.2 | 23 | 0 |
| 66 | 2.8 | 11 | 0 |
| ||||
| 23 | 0 | 22 | 0 |
| ||||
| 42 | 0 | 12 | 0 |
| 25 | 0 | 8 | 0 |
| 25 | 0 | 16 | 0 |
| ||||
| 21 | 0 | 1.4 | 0 |
| 24 | 0 | 14 | 0 |
• ADRs of patients during the 12 Cycle (48 weeks) randomization period. | ||||
Clinically relevant adverse reactions in < 20% of patients who received KOSELUGO versus placebo based on reported adverse reactions included hair changes (18% vs 11%), paronychia (13% vs 4%), pyrexia (7% vs 4%), stomatitis (18% vs 5%), and skin infection (6% vs 1%), respectively. Decreased ejection fraction in patients who received KOSELUGO versus placebo based on reported echocardiogram results occurred in 14% and 11% of patients, respectively.
Table 13 presents the laboratory abnormalities in the KOMET study.
| Randomized to KOSELUGO • (N = 71) | Randomized to Placebo • (N = 74) | ||
All Grades (%) | Grades ≥ 3 (%) | All Grades (%) | Grades ≥ 3 (%) | |
| ||||
Increase creatine phosphokinase | 70 | 7 | 15 | 1.4 |
Increased aspartate aminotransferase (AST) | 48 | 2.9 | 12 | 0 |
Increased alanine aminotransferase (ALT) | 39 | 4.3 | 14 | 0 |
Decreased albumin | 24 | 1.4 | 6 | 0 |
Increased alkaline phosphatase | 17 | 1.4 | 7 | 0 |
Increased amylase | 17 | 1.4 | 5 | 0 |
Decreased magnesium | 16 | 0 | 5 | 0 |
| ||||
Decreased hemoglobin | 24 | 0 | 14 | 0 |
• Lab abnormalities of patients during the 12 Cycle (48 weeks) randomization period. | ||||
Pediatrics > 1 year of Age on KOSELUGO Granules (SPRINKLE)
The safety of KOSELUGO oral granules was evaluated in SPRINKLE (NCT05309668), a dose-finding and activity estimating, single-arm, multicenter study in 36 pediatric patients ages 1 year to less than 7 years with a clinical diagnosis of NF1- related symptomatic, inoperable PN. The study evaluated the pharmacokinetics (PK), safety, efficacy, and tolerability of KOSELUGO oral granules. Study patients were to receive KOSELUGO oral granules for 25 cycles at a dose equivalent to 25 mg/m 2 BSA twice daily until disease progression or unacceptable toxicity. The median age was approximately 4 years (range: 1 to 7 years), 61% were male, 61% were White, 14% were Asian and 3% were Black or African American.
In the SPRINKLE study, the median duration of KOSELUGO oral granules treatment in pediatric patients with neurofibromatosis type 1 (NF1) plexiform neurofibromas (PN) was 11 months (range: 3-25 months). Serious adverse reactions occurred in 6% of patients who received KOSELUGO oral granules. Serious adverse reactions occurred in 1 patient each and included pyrexia, gastroenteritis and upper respiratory infection. A total of 31% of patients had an adverse reaction leading to a dosage interruption. Adverse reactions requiring a dosage interruption in ≥ 5% of patients were pyrexia, vomiting, diarrhea, upper respiratory infection, gastroenteritis and eczema. The most common adverse reactions (≥ 40%) were pyrexia, dry skin, and paronychia.
The observed safety profile of KOSELUGO oral granules in the SPRINKLE study was consistent with the known safety profile of KOSELUGO capsules.
DRUG INTERACTIONS
- Strong or Moderate CYP3A4 Inhibitors or Fluconazole : Avoid coadministration of strong or moderate CYP3A4 inhibitors or fluconazole with KOSELUGO. If coadministration cannot be avoided, reduce the dose of KOSELUGO. (2.5 , 7.1 )
- Strong or Moderate CYP3A4 Inducers : Avoid concomitant use of strong and moderate CYP3A4 inducers. (7.1 )
Effect of Other Drugs on KOSELUGO
Strong or Moderate CYP3A4 Inhibitors or Fluconazole | |
Management |
|
Clinical Impact |
|
Strong or Moderate CYP3A4 Inducers | |
Management |
|
Clinical Impact |
|
Vitamin E | |
Management |
|
Clinical Impact |
|
DESCRIPTION
KOSELUGO contains selumetinib sulfate, a kinase inhibitor. The chemical name is 5-[(4-bromo-2-chlorophenyl)amino]-4-fluoro-6-[(2-hydroxyethoxy)carbamoyl]-1-methyl-1 H -benzimidazol-3-ium hydrogen sulfate. The molecular formula for selumetinib sulfate is C 17 H 17 BrClFN 4 O 7 S and the relative molecular mass is 555.76 g/mol. Selumetinib sulfate has the following structural formula:

Selumetinib sulfate is a white to yellow monomorphic crystalline powder that exhibits a pH dependent solubility. Selumetinib sulfate is freely soluble at pH < 1.5, sparingly soluble in the pH range at 1.5 to 3 and slightly soluble at pH > 3. Selumetinib sulfate has two ionizable functions with pKa values of 2.8 and 8.4.
KOSELUGO (selumetinib) 10 mg capsules for oral use, contain 10 mg selumetinib (equivalent to 12.1 mg selumetinib sulfate) and the excipient, vitamin E polyethylene glycol succinate. The capsule shell contains carnauba wax, carrageenan, hypromellose, potassium chloride, purified water, and titanium dioxide. The capsule is imprinted with black ink that contains ammonium hydroxide, iron oxide black, propylene glycol, and shellac.
KOSELUGO (selumetinib) 25 mg capsules for oral use, contain 25 mg selumetinib (equivalent to 30.25 mg selumetinib sulfate) and the excipient, vitamin E polyethylene glycol succinate. The capsule shell contains carnauba wax and/or cornstarch, carrageenan, FD&C blue 2, ferric oxide yellow, hypromellose, potassium chloride, purified water, and titanium dioxide. The capsule is imprinted with black ink that contains carnauba wax, FD&C Blue 2 aluminum lake, ferric oxide red, ferric oxide yellow, glyceryl monooleate, and shellac.
KOSELUGO (selumetinib) 5 mg oral granules contain 5 mg selumetinib (equivalent to 6.05 mg selumetinib sulfate). The uncoated cores contain selumetinib sulfate, glyceryl dibehenate, and stearoyl polyoxylglycerides. The granule coating contains acetone, hypromellose acetate succinate, and stearic acid. The capsule shell contains ferric oxide yellow, hypromellose, and titanium dioxide. The capsule shell is imprinted with black ink that contains butyl alcohol, dehydrated alcohol, ferric oxide black, isopropyl alcohol, potassium hydroxide, propylene glycol, purified water, shellac, and strong ammonia solution.
KOSELUGO (selumetinib) 7.5 mg oral granules contain 7.5 mg selumetinib (equivalent to 9.08 mg selumetinib sulfate). The uncoated cores contain selumetinib sulfate, glyceryl dibehenate, and stearoyl polyoxylglycerides. The granule coating contains acetone, hypromellose acetate succinate, and stearic acid. The capsule shell contains ferric oxide red, hypromellose, and titanium dioxide. The capsule shell is imprinted with black ink that contains butyl alcohol, dehydrated alcohol, ferric oxide black, isopropyl alcohol, potassium hydroxide, propylene glycol, purified water, shellac, and strong ammonia solution.
CLINICAL PHARMACOLOGY
Mechanism of Action
Selumetinib is an inhibitor of mitogen-activated protein kinase kinases 1 and 2 (MEK1/2). MEK1/2 proteins are upstream regulators of the extracellular signal-related kinase (ERK) pathway. Both MEK and ERK are critical components of the RAS-regulated RAF-MEK-ERK pathway, which is often activated in different types of cancers.
In genetically modified mouse models of NF1 that generate neurofibromas that recapitulate the genotype and phenotype of human NF1, oral dosing of selumetinib inhibited ERK phosphorylation, and reduced neurofibroma numbers, volume, and proliferation.
Pharmacodynamics
The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of KOSELUGO have not been fully characterized.
Cardiac Electrophysiology
At a dose 1.5-times the maximum recommended dose, clinically significant QTc interval prolongation was not observed.
Pharmacokinetics
Selumetinib pharmacokinetics were observed at steady state in adult and pediatric patients with NF1 and are presented as mean (CV%) unless otherwise indicated.
The maximum plasma concentration (C max ) is 792 (49) ng/mL and systemic exposure (AUC) is 2141 (55) ng•h/mL following KOSELUGO 25 mg/m 2 twice daily. Selumetinib AUC and C max increases in a dose proportional manner over a dose range from 20 mg/m 2 to 30 mg/m 2 (0.8 to 1.2 times the recommended dose).
Selumetinib accumulation range is 1.2-1.5 fold following administration of KOSELUGO at 25 mg/m 2 .
At the recommended dosage of 25 mg/m 2 of KOSELUGO oral granules (sprinkled on smooth yogurt, smooth fruit sauce, smooth fruit puree, or smooth fruit jam) twice daily in pediatric patients (> 1 year old to < 7 years old), the AUC 0-24h following the first dose of KOSELUGO oral granules was within the range of that in patients administered KOSELUGO capsules.
No clinically relevant differences in the pharmacokinetics of selumetinib were observed following administration of a single-dose of either the granule or capsule dosage forms of KOSELUGO at equivalent dosages, under fasted and fed conditions, in healthy adults.
Absorption
Selumetinib absolute oral bioavailability is 62%. Selumetinib median (min, max) time to maximum plasma concentrations (Tmax) is 1.5 (0.22, 6.0) hours.
Effect of Food
No clinically significant differences in selumetinib pharmacokinetics were observed following administration of low fat (400-500 calories) or high-fat (800-1000 calories) meal.
Distribution
Selumetinib apparent volume of distribution across a dose range of 20 mg/m 2 to 30 mg/m 2 (0.8 to 1.2 times the recommended dosage) ranges from 40 L-3710 L (66).
The plasma protein binding is 98.4% (primarily albumin).
Elimination
Selumetinib elimination half-life is 9 (28) hours with an apparent (oral) clearance of 16 (44) L/hr/m 2 .
Metabolism
Selumetinib is primarily metabolized by CYP3A4 and to a lesser extent by CYP2C19, CYP1A2, CYP2C9, CYP2E1, and CYP3A5. Selumetinib also undergoes glucuronidation by UGT1A1 and UGT1A3. It is estimated that 56% of the observed intrinsic clearance of selumetinib can be attributed to CYP metabolism and about 29% to direct glucuronidation by UGT enzymes.
The active metabolite, N desmethyl selumetinib, is generated by CYP2C19 and CYP1A2 with additional contribution by CYP2C9 and CYP2A6, and it is metabolized through the same routes as selumetinib.
N-desmethyl selumetinib represents < 10% of selumetinib levels in human plasma, but is approximately 3- to 5- times more potent than the parent compound, contributing to about 21% to 35% of the overall pharmacologic activity.
Excretion
After a single oral dose of radiolabeled selumetinib 75 mg (1.5-times the recommended dose) to healthy adults, 59% of the dose was recovered in feces (19% as unchanged) and 33% in urine (< 1% as parent).
Specific Populations
No clinically significant differences in the pharmacokinetics of selumetinib or N-desmethyl selumetinib were observed based on age (1-79 years) race [White (56%), Asian (22%), Black (19%)].
Pediatric Patients
No clinically significant differences in the pharmacokinetics of selumetinib or N-desmethyl selumetinib were observed between the pediatric (aged 1 to < 17 years) and adult patients.
Patients with Renal Impairment
No clinically significant differences in selumetinib exposures were observed in patients with end stage renal disease (CLcr < 15 mL/min) who required dialysis. CLcr was estimated using the Cockroft-Gault formula.
Patients with Hepatic Impairment
Dose normalized total AUC increased by 1.6-fold in patients with moderate hepatic impairment (Child-Pugh B) and in patients with severe hepatic impairment (Child-Pugh class C). Selumetinib unbound AUC increased by 1.4-fold in patients with moderate hepatic impairment (Child-Pugh B), and 3.2-fold in patients with severe hepatic impairment (Child-Pugh C).
Drug Interaction Studies
Clinical Studies and Model-Informed Approaches
Strong or Moderate CYP3A4 Inhibitors: Selumetinib AUC increased by 1.5-fold and C max by 1.2-fold following concomitant administration of itraconazole (strong CYP3A4 inhibitor). Concomitant use of erythromycin (moderate CYP3A4 inhibitor) is predicted to increase selumetinib AUC by 1.4-fold and C max by 1.2-fold.
Fluconazole: Selumetinib AUC increased by 1.5 fold and C max by 1.3 fold following concomitant administration of fluconazole (strong CYP2C19 inhibitor and moderate CYP3A4 inhibitor).
Strong or Moderate CYP3A4 Inducers: Selumetinib AUC decreased by 51% and C max by 26% following concomitant administration of rifampicin (strong CYP3A4 inducer). Concomitant use of efavirenz (moderate CYP3A4 inducer) is predicted to decrease selumetinib AUC by 38% and C max by 22%.
In Vitro Studies
CYP Enzymes: Selumetinib does not inhibit CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP3A4, or CYP2E1. Selumetinib does not induce CYP3A4, CYP1A2, or CYP2B6.
Transporter Systems: Selumetinib does not inhibit BCRP, P-glycoprotein (P-gp), OATP1B1, OATP1B3, OCT2, OAT1, OAT3, MATE1, or MATE2K.
Selumetinib is a substrate of BCRP and P-gp.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
Selumetinib was not carcinogenic in a 6-month study in rasH2 transgenic mice and in 2-year carcinogenicity study in rats at exposures ≥ 15 times the human exposure (AUC) at the clinical dose of 25 mg/m 2 .
Mutagenicity
Selumetinib was not mutagenic or clastogenic in vitro . Selumetinib did result in an increase in micronucleated immature erythrocytes (chromosome aberrations) in mouse micronucleus studies, predominantly via an aneugenic mode of action, but at doses > 160 mg/kg (~38-times the human C max at the clinical dose of 25 mg/m 2 ).
Impairment of Fertility
In a 6-month mouse study, selumetinib did not affect male mating performance at any dose up to 20 mg/kg twice daily (approximately 33-times the human exposure based on AUC at the clinical dose of 25 mg/m 2 twice daily). In female mice exposed to selumetinib at 12.5 mg/kg twice daily, mating performance and fertility were not affected. The NOAEL for both maternal toxicity and effects on reproductive performance was 2.5 mg/kg twice daily (approximately 5-times the human exposure based on AUC at the clinical dose of 25 mg/m 2 twice daily).
Animal Toxicology and/or Pharmacology
In a 26-week repeat-dose toxicology study, selumetinib at a dose of 20 mg/kg (approximately 33-times the human exposure based on AUC at the clinical dose of 25 mg/m 2 twice daily) led to significant urinary tract obstruction as well as inflammation and luminal hemorrhage of the urethra leading to early death in male mice.
CLINICAL STUDIES
Neurofibromatosis Type 1 (NF1) with Inoperable Plexiform Neurofibromas (PN)
Pediatrics 2 18 years of Age (SPRINT Phase II Stratum 1)
The efficacy of KOSELUGO was evaluated in SPRINT Phase II Stratum 1, an-open label, multicenter, single arm trial (NCT01362803). Eligible patients were required to have NF1 with inoperable PN, defined as a PN that could not be completely removed without risk for substantial morbidity due to encasement of, or close proximity to, vital structures, invasiveness, or high vascularity of the PN. Patients were also required to have significant morbidity related to the target PN. Morbidities that were present in > 20% of patients included disfigurement, motor dysfunction, pain, airway dysfunction, visual impairment, and bladder/bowel dysfunction. Patients received KOSELUGO 25 mg/m 2 orally twice daily until disease progression or unacceptable toxicity.
The major efficacy outcome measure was overall response rate (ORR), defined as the percentage of patients with complete response (defined as disappearance of the target PN) or confirmed partial response (defined as ≥ 20% reduction in PN volume confirmed at a subsequent tumor assessment within 3-6 months). The target PN, defined as the PN that caused relevant clinical symptoms or complications (PN-related morbidities), was evaluated for response rate using centrally read volumetric magnetic resonance imaging (MRI) analysis per Response Evaluation in Neurofibromatosis and Schwannomatosis (REiNS) criteria. Tumor response was evaluated at baseline and while on treatment after every 4 cycles for 2 years, and then every 6 cycles. An additional efficacy outcome measure was duration of response (DoR).
A total of 50 pediatric patients received KOSELUGO. The median age was 10.2 years (range 3.5 to 17.4 years); 60% were male; and 84% were White, 8% were Black and 2% were Asian.
Efficacy results are provided in Table 14. The median time to onset of response was 7.2 months (range: 3.3 months to 1.6 years).
| Efficacy Parameter | SPRINT N = 50 |
|---|---|
Overall Response Rate Responses required confirmation at least 3 months after the criteria for first response were met. The ORR assessment (data cut-off date [DCO]: June 2018) was conducted by a single National Cancer Institute reviewer who was a SPRINT investigator and who evaluated all PN imaging from patients enrolled at all trial sites. | |
Overall Response Rate, n (%) | 33 (66%) |
95% CI | (51, 79) |
Complete Response Complete response: disappearance of the target lesion; Partial response: decrease in target PN volume by ≥ 20% compared to baseline. | 0 |
Confirmed Partial Response, n (%) | 33 (66%) |
Duration of Response DCO: March 2021. | |
Median (95% CI) months | NR (41.2 – NE) |
DoR ≥ 24 months, n (%) | 26 (79%) |
DoR ≥ 36 months, n (%) | 21 (64%) |
CI – confidence interval, DoR – duration of response, NE – not evaluable, NR – not reached. | |
An independent centralized review of tumor response per REiNS criteria (data cut-off June 2018) resulted in an ORR of 44% (95% CI: 30, 59).
Adults ≥ 18 years of Age (KOMET)
The efficacy of KOSELUGO in adult patients was evaluated in KOMET, a randomized, multicenter, double-blind, placebo-controlled trial (NCT04924608). Eligible patients were required to be 18 years of age or older with neurofibromatosis type 1 (NF1) and symptomatic, inoperable plexiform neurofibroma (PN). Inoperable plexiform neurofibroma (PN) is defined as a PN that could not be completely removed without risk for substantial morbidity due to encasement of, or close proximity to, vital structures, invasiveness, or high vascularity of the PN.
A total of 145 patients were randomized (1:1) to KOSELUGO 25 mg/m 2 (BSA) or placebo twice daily for 12 cycles (28-day cycles). After the end of Cycle 12, or earlier if disease progression was confirmed by the Independent Central Review (ICR), patients initially randomized to placebo crossed over to receive KOSELUGO in the open-label treatment phase. Treatment was discontinued if a patient was no longer deriving clinical benefit, experienced unacceptable toxicity, patient decision, PN progression, or at the discretion of the investigator.
One target and when applicable one non-target PN was assessed by using volumetric MRI analysis and REiNS criteria.
The major efficacy outcome measure was overall response rate (ORR) by the end of Cycle 16, as determined by ICR per REiNS criteria. Duration of response was an additional efficacy outcome measure.
The study demographics were 52% male, 56% White, 31% Asian, 6% Black or African American, 10% Hispanic or Latino ethnicity, and the median age was 29 years (range: 18 to 60 years). PN-related morbidities that were present in > 20% of patients included pain, motor dysfunction, and disfigurement.
The trial demonstrated statistically significant ORR in patients randomized to KOSELUGO compared to placebo by the end of Cycle 16. The median time to response was 3.7 months (range: 3.6 months to 11.2 months).
Efficacy results are shown in Table 15.
Efficacy Parameters | KOSELUGO (N = 71) | Placebo (N = 74) |
Overall Response Rate by the end of Cycle 16 (ORR) Patients with confirmed complete response or partial response by independent central review (ICR) per REiNS criteria. Response confirmation was by a consecutive scan within 3 to 6 months after the first response as determined by ICR per REiNS criteria. | ||
ORR % (95% CI) All.Partial responders. | 20 (11, 31) | 5 (2, 13) |
p value 2-sided p-value calculated using Fisher’s exact method. | 0.011 | |
Duration of Response Calculated using Kaplan-Meier method. | ||
Median (95% CI) months | NR (11.5, NE) | ND |
≥ 6 months, n (%) | 12 (86%) | ND |
CI – confidence interval, ND – Not determined for placebo arm, NE – not estimable, NR - not reached. | ||
HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Selumetinib Capsules
Strength | Description | Capsules per Bottle | NDC Number |
10 mg | White to off-white, opaque, hard capsule sealed with a clear band and marked with “SEL 10” in black ink. | 60 | 0310-0610-60 |
28 | 0310-0610-28 | ||
25 mg | Blue, opaque, hard capsule sealed with a clear band and marked with “SEL 25” in black ink. | 60 | 0310-0625-60 |
28 | 0310-0625-28 |
Selumetinib Oral Granules
| Strength | Description | Capsules per Bottle | NDC Number |
|---|---|---|---|
5 mg | Off‑white to light‑yellow free‑flowing oral granules contained within capsules. The capsules have a yellow cap and white body. The cap is printed with “sel 5” in black ink, and body is printed with a sprinkle capsule image indicating opening. | 60 | 0310‑0635‑60 |
7.5 mg | Off‑white to light‑yellow free‑flowing oral granules contained within capsules. The capsules have a pink cap and white body where the cap is printed with “sel 7.5” in black ink, and body is printed with a sprinkle capsule image indicating opening. | 60 | 0310‑0640‑60 |
Storage
KOSELUGO Capsules
Store KOSELUGO capsules at 20°C to 25°C (68°F to 77°F) with excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Dispense and store in the original bottle to protect from light and moisture. Do not remove desiccant. Keep the bottle tightly closed after first opening.
KOSELUGO Oral Granules
Store and transport KOSELUGO oral granules refrigerated at 2°C to 8°C (36°F to 46°F). After receipt, patients may store at room temperature 20°C to 25°C (68°F to 77°F). Do NOT exceed 30°C (86°F). KOSELUGO oral granules may clump together or stick to the capsule shell if exposed to high temperatures, which may lead to underdose. Dispense and store in the original bottle to protect from light and moisture. Do not remove desiccant. Keep the bottle tightly closed after first opening.
Instructions for Use
INSTRUCTIONS FOR USE KOSELUGO ® (ko-SEL-u-go) (selumetinib) oral granules | |
This Instructions for Use contains information on how to take or give KOSELUGO oral granules. Read this Instructions for Use before taking or giving KOSELUGO oral granules and each time you get a refill. There may be new information . This information does not take the place of talking to your healthcare provider about your or your child’s medical condition or treatment. Talk to your healthcare provider or pharmacist if you have any questions about how to take or give KOSELUGO oral granules. | |
Important Information You Need to Know Before Taking or Giving KOSELUGO oral granules
Supplies needed to prepare a dose of KOSELUGO oral granules :
| |
Preparing KOSELUGO oral granules | |
Step 1: Wash and dry your hands thoroughly. | 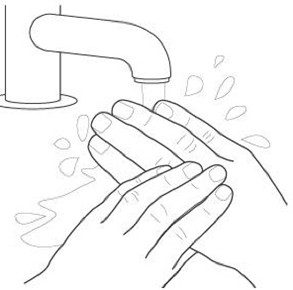 |
Step 2: Open the bottle(s) and take out the number of capsule(s) needed for your prescribed dose of KOSELUGO oral granules. | 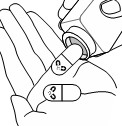 |
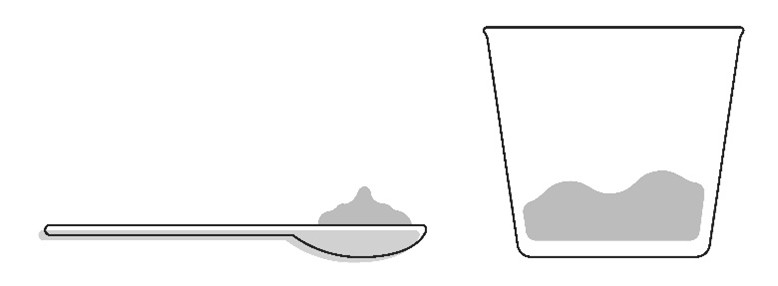
Step 3: Add a small amount (about 1 to 3 teaspoons) of smooth yogurt, or fruit puree containing apple, banana, pear, or strawberry onto the spoon or into a small cup.
|  |
Step 4: Place the spoon or cup on the paper towel or plate. Carefully open the capsule(s) one at a time and sprinkle the entire contents of the capsule (oral granules) onto the food that is on the spoon or in the small cup.
| 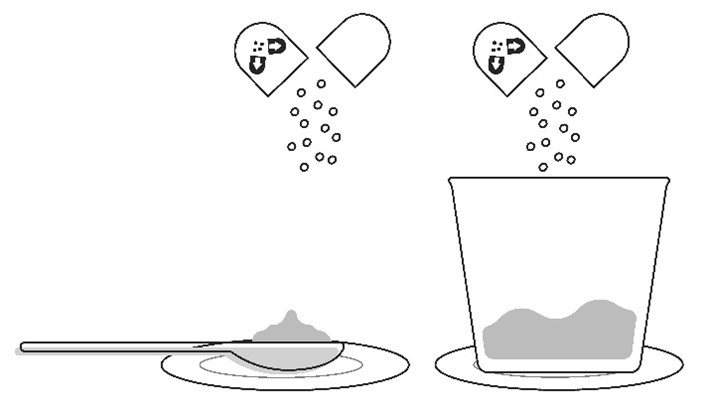 Important: The oral granules should flow freely inside the capsule. Do not use the capsule if the oral granules are clumped or stuck inside the capsule shell. Contact your pharmacy if this happens. |
Taking or giving KOSELUGO oral granules | |
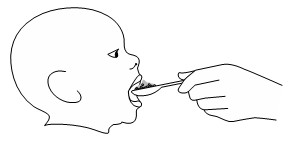
Step 5: Take or give the KOSELUGO oral granules and food mixture within 30 minutes of preparing the dose. Swallow the mixture without chewing while seated upright. Do not save for future use. Note:
|  |
Step 6: If you used a cup to prepare KOSELUGO oral granules, add more of the same food into the cup and take or give all of the mixture to make sure you get the full dose. | 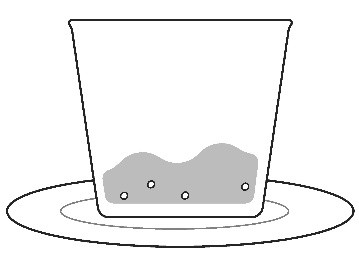 |
Clean up
| |
Storing KOSELUGO oral granules
Keep KOSELUGO and all medicines out of the reach of children. | |
Disposing of KOSELUGO oral granules
| |
Distributed by: AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850 ©AstraZeneca 2025 For more information, go to website www.KOSELUGO.com or call 1-800-236-9933. | |
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Approved: Sep/2025
Mechanism of Action
Selumetinib is an inhibitor of mitogen-activated protein kinase kinases 1 and 2 (MEK1/2). MEK1/2 proteins are upstream regulators of the extracellular signal-related kinase (ERK) pathway. Both MEK and ERK are critical components of the RAS-regulated RAF-MEK-ERK pathway, which is often activated in different types of cancers.
In genetically modified mouse models of NF1 that generate neurofibromas that recapitulate the genotype and phenotype of human NF1, oral dosing of selumetinib inhibited ERK phosphorylation, and reduced neurofibroma numbers, volume, and proliferation.