Leucovorin Calcium
Leucovorin Calcium Prescribing Information
Leucovorin calcium rescue is indicated after high dose methotrexate therapy in osteosarcoma. Leucovorin Calcium for Injection is also indicated to diminish the toxicity and counteract the effects of impaired methotrexate elimination and of inadvertent overdosages of folic acid antagonists.
Leucovorin Calcium for Injection is indicated in the treatment of megaloblastic anemias due to folic acid deficiency when oral therapy is not feasible.
Leucovorin Calcium for Injection is also indicated for use in combination with 5-fluorouracil to prolong survival in the palliative treatment of patients with advanced colorectal cancer. Leucovorin Calcium for Injection should not be mixed in the same infusion as 5-fluorouracil because a precipitate may form.
Either of the following two regimens is recommended:
- Leucovorin calcium for injection is administered at 200 mg/m2 by slow intravenous injection over a minimum of 3 minutes, followed by 5-fluorouracil at 370 mg/m2 by intravenous injection.
- Leucovorin calcium for injection is administered at 20 mg/m2 by intravenous injection followed by 5-fluorouracil at 425 mg/m2 by intravenous injection.
5-Fluorouracil and leucovorin should be administered separately to avoid the formation of a precipitate.
Treatment is repeated daily for five days. This five-day treatment course may be repeated at 4 week (28-day) intervals, for 2 courses and then repeated at 4 to 5 week (28 to 35 day) intervals provided that the patient has completely recovered from the toxic effects of the prior treatment course.
In subsequent treatment courses, the dosage of 5-fluorouracil should be adjusted based on patient tolerance of the prior treatment course. The daily dosage of 5-fluorouracil should be reduced by 20% for patients who experienced moderate hematologic or gastrointestinal toxicity in the prior treatment course, and by 30% for patients who experienced severe toxicity (see
Patients being treated with the leucovorin/5-fluorouracil combination should have a CBC with differential and platelets prior to each treatment. During the first two courses a CBC with differential and platelets has to be repeated weekly and thereafter once each cycle at the time of anticipated WBC nadir. Electrolytes and liver function tests should be performed prior to each treatment for the first three cycles then prior to every other cycle. Dosage modifications of fluorouracil should be instituted as follows, based on the most severe toxicities:
| Diarrhea and/or Stomatitis | WBC/mm3 Nadir | Platelets/mm3 Nadir | 5-FU Dose |
| Moderate | 1,000 to 1,900 | 25 to 75,000 | decrease 20% |
| Severe | < 1,000 | < 25,000 | decrease 30% |
If no toxicity occurs, the 5-fluorouracil dose may increase 10%. Treatment should be deferred until WBCs are 4,000/mm3and platelets 130,000/mm3. If blood counts do not reach these levels within two weeks, treatment should be discontinued. Patients should be followed up with physical examination prior to each treatment course and appropriate radiological examination as needed. Treatment should be discontinued when there is clear evidence of tumor progression.
Leucovorin is improper therapy for pernicious anemia and other megaloblastic anemias secondary to the lack of vitamin B12. A hematologic remission may occur while neurologic manifestations continue to progress.
Allergic sensitization, including anaphylactoid reactions and urticaria, has been reported following the administration of both oral and parenteral leucovorin. No other adverse reactions have been attributed to the use of leucovorin
The following table summarizes significant adverse events occurring in 316 patients treated with the leucovorin/5-fluorouracil combinations compared against 70 patients treated with 5-fluorouracil alone for advanced colorectal carcinoma. These data are taken from the Mayo/NCCTG large multicenter prospective trial evaluating the efficacy and safety of the combination regimen.
High LV = Leucovorin 200 mg/m2, Low LV = Leucovorin 20 mg/m2 | ||||||
Any = percentage of patients reporting toxicity of any severity | ||||||
Grade 3+ = percentage of patients reporting toxicity of Grade 3 or higher | ||||||
| (High LV)/5-FU (N=155) | (Low LV)/5-FU (N=161) | 5-FU Alone (N=70) | ||||
| Any | Grade 3+ | Any | Grade 3+ | Any | Grade 3+ | |
| (%) | (%) | (%) | (%) | (%) | (%) | |
| Leukopenia | 69 | 14 | 83 | 23 | 93 | 48 |
| Thrombocytopenia | 8 | 2 | 8 | 1 | 18 | 3 |
| Infection | 8 | 1 | 3 | 1 | 7 | 2 |
| Nausea | 74 | 10 | 80 | 9 | 60 | 6 |
| Vomiting | 46 | 8 | 44 | 9 | 40 | 7 |
| Diarrhea | 66 | 18 | 67 | 14 | 43 | 11 |
| Stomatitis | 75 | 27 | 84 | 29 | 59 | 16 |
| Constipation | 3 | 0 | 4 | 0 | 1 | - |
| Lethargy/Malaise/Fatigue | 13 | 3 | 12 | 2 | 6 | 3 |
| Alopecia | 42 | 5 | 43 | 6 | 37 | 7 |
| Dermatitis | 21 | 2 | 25 | 1 | 13 | - |
| Anorexia | 14 | 1 | 22 | 4 | 14 | - |
| Hospitalization for Toxicity | 5% | 15% | 7% | |||
Folic acid in large amounts may counteract the antiepileptic effect of phenobarbital, phenytoin and primidone, and increase the frequency of seizures in susceptible pediatric patients.
Preliminary animal and human studies have shown that small quantities of systemically administered leucovorin enter the CSF primarily as 5-methyltetrahydrofolate and, in humans, remain 1 to 3 orders of magnitude lower than the usual methotrexate concentrations following intrathecal administration. However, high doses of leucovorin may reduce the efficacy of intrathecally administered methotrexate.
Leucovorin may enhance the toxicity of 5-fluorouracil (see
In the treatment of accidental overdosages of folic acid antagonists, intravenous leucovorin should be administered as promptly as possible. As the time interval between antifolate administration (e.g., methotrexate) and leucovorin rescue increases, leucovorin's effectiveness in counteracting toxicity decreases. In the treatment of accidental overdosages of intrathecally administered folic acid antagonists, do not administer leucovorin intrathecally. LEUCOVORIN MAY BE HARMFUL OR FATAL IF GIVEN INTRATHECALLY.
Monitoring of the serum methotrexate concentration is essential in determining the optimal dose and duration of treatment with leucovorin.
Delayed methotrexate excretion may be caused by a third space fluid accumulation (i.e., ascites, pleural effusion), renal insufficiency, or inadequate hydration. Under such circumstances, higher doses of leucovorin or prolonged administration may be indicated. Doses higher than those recommended for oral use must be given intravenously.
Because of the benzyl alcohol contained in certain diluents used for reconstituting leucovorin, when doses greater than 10 mg/m2are administered, leucovorin should be reconstituted with Sterile Water for Injection, USP, and used immediately (see
Because of the calcium content of the leucovorin solution, no more than 160 mg of leucovorin should be injected intravenously per minute (16 mL of a 10 mg/mL, or 8 mL of a 20 mg/mL solution per minute).
Leucovorin enhances the toxicity of 5-fluorouracil. When these drugs are administered concurrently in the palliative therapy of advanced colorectal cancer, the dosage of 5-fluorouracil must be lower than usually administered. Although the toxicities observed in patients treated with the combination of leucovorin plus 5-fluorouracil are qualitatively similar to those observed in patients treated with 5-fluorouracil alone, gastrointestinal toxicities (particularly stomatitis and diarrhea) are observed more commonly and may be more severe and of prolonged duration in patients treated with the combination.
In the first Mayo/NCCTG controlled trial, toxicity, primarily gastrointestinal, resulted in 7% of patients requiring hospitalization when treated with 5-fluorouracil alone or 5-fluorouracil in combination with 200 mg/m2of leucovorin and 20% when treated with 5-fluorouracil in combination with 20 mg/m2of leucovorin. In the second Mayo/NCCTG trial, hospitalizations related to treatment toxicity also appeared to occur more often in patients treated with the low dose leucovorin/5-fluorouracil combination than in patients treated with the high dose combination — 11% versus 3%. Therapy with leucovorin and 5-fluorouracil must not be initiated or continued in patients who have symptoms of gastrointestinal toxicity of any severity, until those symptoms have completely resolved. Patients with diarrhea must be monitored with particular care until the diarrhea has resolved, as rapid clinical deterioration leading to death can occur. In an additional study utilizing higher weekly doses of 5-fluorouracil and leucovorin, elderly and/or debilitated patients were found to be at greater risk for severe gastrointestinal toxicity.
Seizures and/or syncope have been reported rarely in cancer patients receiving leucovorin, usually in association with fluoropyrimidine administration, and most commonly in those with CNS metastases or other predisposing factors, however, a causal relationship has not been established.
The concomitant use of leucovorin with trimethoprim-sulfamethoxazole for the acute treatment of Pneumocystis carinii pneumonia in patients with HIV infection was associated with increased rates of treatment failure and morbidity in a placebo-controlled study.
Leucovorin is one of several active, chemically reduced derivatives of folic acid. It is useful as an antidote to drugs which act as folic acid antagonists.
Also known as folinic acid, Citrovorum factor, or 5-formyl-5,6,7,8-tetrahydrofolic acid, this compound has the chemical designation of Calcium
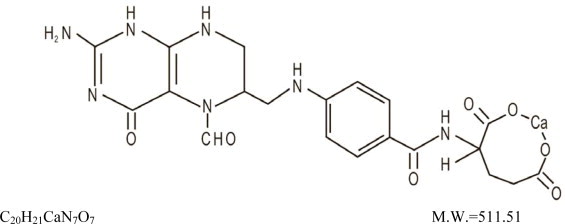
Leucovorin Calcium for Injection, USP is a sterile product indicated for intramuscular (IM) or intravenous (IV) administration and is supplied in 50 mg, 100 mg, 200 mg, and 350 mg vials.
Each 50 mg vial of Leucovorin Calcium for Injection, USP, when reconstituted with 5 mL of sterile diluent, contains leucovorin (as the calcium salt) 10 mg per mL.
Each 100 mg vial of Leucovorin Calcium for Injection, USP, when reconstituted with 10 mL of sterile diluent, contains leucovorin (as the calcium salt) 10 mg per mL.
Each 200 mg vial of Leucovorin Calcium for Injection, USP, when reconstituted with 20 mL of sterile diluent, contains leucovorin (as the calcium salt) 10 mg per mL.
Each 350 mg vial of Leucovorin Calcium for Injection, USP, when reconstituted with 17.5 mL of sterile diluent, contains leucovorin (as the calcium salt) 20 mg per mL.
In each dosage form, one milligram of leucovorin calcium contains 0.002 mmol of leucovorin and 0.002 mmol of calcium.
These lyophilized products contain no preservative. The inactive ingredient is Sodium Chloride, USP, added to adjust tonicity. Sodium hydroxide and / or hydrochloric acid may be used to adjust the pH. The pH is between 6.5 and 8.5. Reconstitute with Bacteriostatic Water for Injection, USP, which contains benzyl alcohol (see
In the treatment of accidental overdosages of folic acid antagonists, intravenous leucovorin should be administered as promptly as possible. As the time interval between antifolate administration (e.g., methotrexate) and leucovorin rescue increases, leucovorin's effectiveness in counteracting toxicity decreases. In the treatment of accidental overdosages of intrathecally administered folic acid antagonists, do not administer leucovorin intrathecally. LEUCOVORIN MAY BE HARMFUL OR FATAL IF GIVEN INTRATHECALLY.
Monitoring of the serum methotrexate concentration is essential in determining the optimal dose and duration of treatment with leucovorin.
Delayed methotrexate excretion may be caused by a third space fluid accumulation (i.e., ascites, pleural effusion), renal insufficiency, or inadequate hydration. Under such circumstances, higher doses of leucovorin or prolonged administration may be indicated. Doses higher than those recommended for oral use must be given intravenously.
Because of the benzyl alcohol contained in certain diluents used for reconstituting leucovorin, when doses greater than 10 mg/m2are administered, leucovorin should be reconstituted with Sterile Water for Injection, USP, and used immediately (see
Because of the calcium content of the leucovorin solution, no more than 160 mg of leucovorin should be injected intravenously per minute (16 mL of a 10 mg/mL, or 8 mL of a 20 mg/mL solution per minute).
Leucovorin enhances the toxicity of 5-fluorouracil. When these drugs are administered concurrently in the palliative therapy of advanced colorectal cancer, the dosage of 5-fluorouracil must be lower than usually administered. Although the toxicities observed in patients treated with the combination of leucovorin plus 5-fluorouracil are qualitatively similar to those observed in patients treated with 5-fluorouracil alone, gastrointestinal toxicities (particularly stomatitis and diarrhea) are observed more commonly and may be more severe and of prolonged duration in patients treated with the combination.
In the first Mayo/NCCTG controlled trial, toxicity, primarily gastrointestinal, resulted in 7% of patients requiring hospitalization when treated with 5-fluorouracil alone or 5-fluorouracil in combination with 200 mg/m2of leucovorin and 20% when treated with 5-fluorouracil in combination with 20 mg/m2of leucovorin. In the second Mayo/NCCTG trial, hospitalizations related to treatment toxicity also appeared to occur more often in patients treated with the low dose leucovorin/5-fluorouracil combination than in patients treated with the high dose combination — 11% versus 3%. Therapy with leucovorin and 5-fluorouracil must not be initiated or continued in patients who have symptoms of gastrointestinal toxicity of any severity, until those symptoms have completely resolved. Patients with diarrhea must be monitored with particular care until the diarrhea has resolved, as rapid clinical deterioration leading to death can occur. In an additional study utilizing higher weekly doses of 5-fluorouracil and leucovorin, elderly and/or debilitated patients were found to be at greater risk for severe gastrointestinal toxicity.
Seizures and/or syncope have been reported rarely in cancer patients receiving leucovorin, usually in association with fluoropyrimidine administration, and most commonly in those with CNS metastases or other predisposing factors, however, a causal relationship has not been established.
The concomitant use of leucovorin with trimethoprim-sulfamethoxazole for the acute treatment of Pneumocystis carinii pneumonia in patients with HIV infection was associated with increased rates of treatment failure and morbidity in a placebo-controlled study.