Lidocaine Hydrochloride
Lidocaine Hydrochloride Prescribing Information
Lidocaine Hydrochloride Oral Topical Solution, USP 2% (Viscous) is indicated for the production of topical anesthesia of irritated or inflamed mucous membranes of the mouth and pharynx. It is also useful for reducing gagging during the taking of X-ray pictures and dental impressions.
The maximum recommended single dose of Lidocaine Hydrochloride Oral Topical Solution, USP 2% (Viscous) for healthy adults should be such that the dose of lidocaine HCl does not exceed 4.5 mg/kg or 2 mg/lb body weight and does not in any case exceed a total of 300 mg.
For symptomatic treatment of irritated or inflamed mucous membranes of the mouth and pharynx, the usual adult dose is 15 mL undiluted. For use in the mouth, the solution should be swished around in the mouth and spit out. For use in the pharynx, the undiluted solution should be gargled and may be swallowed. This dose should not be administered at intervals of less than three hours, and not more than eight doses should be given in a 24-hour period. The dosage should be adjusted commensurate with the patient's age, weight and physical condition (see
Care must be taken to ensure correct dosage in all pediatric patients as there have been cases of overdose due to inappropriate dosing.
It is difficult to recommend a maximum dose of any drug for children since this varies as a function of age and weight. For children over 3 years of age who have a normal lean body mass and normal body development, the maximum dose is determined by the child's weight or age. For example: in a child of 5 years weighing 50 lbs., the dose of lidocaine hydrochloride should not exceed 75 to 100 mg (3.7 to 5 mL of Lidocaine Hydrochloride Oral Topical Solution, USP 2% (Viscous)).
For infants and in children under 3 years of age, the solution should be accurately measured and no more than 1.2 mL be applied to the immediate area with a cotton-tipped applicator. Wait at least 3 hours before giving the next dose; a maximum of four doses may be given in a 12-hour period. Lidocaine Hydrochloride Oral Topical Solution, USP 2% (Viscous) should only be used if the underlying condition requires treatment with a volume of product that is less than or equal to 1.2 mL.
Lidocaine is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type, or to other components of the solution.
Adverse experiences following the administration of lidocaine are similar in nature to those observed with other amide local anesthetic agents. These adverse experiences are, in general, dose-related and may result from high plasma levels caused by excessive dosage or rapid absorption, or may result from a hypersensitivity, idiosyncrasy or diminished tolerance on the part of the patient. Serious adverse experiences are generally systemic in nature. The following types are those most commonly reported:
CNS manifestations are excitatory and/or depressant and may be characterized by lightheadedness, nervousness, apprehension, euphoria, confusion, dizziness, drowsiness, tinnitus, blurred or double vision, vomiting, sensations of heat, cold or numbness, twitching, tremors, convulsions, unconsciousness, respiratory depression and arrest. The excitatory manifestations may be very brief or may not occur at all, in which case the first manifestation of toxicity may be drowsiness merging into unconsciousness and respiratory arrest.
Drowsiness following the administration of lidocaine is usually an early sign of a high blood level of the drug and may occur as a consequence of rapid absorption.
Cardiovascular manifestations are usually depressant and are characterized by bradycardia, hypotension, and cardiovascular collapse, which may lead to cardiac arrest.
Allergic reactions are characterized by cutaneous lesions, urticaria, edema or anaphylactoid reactions. Allergic reactions may occur as a result of sensitivity either to the local anesthetic agent or to the methylparaben and/or propylparaben used in this formulation. Allergic reactions as a result of sensitivity to lidocaine are extremely rare and, if they occur, should be managed by conventional means. The detection of sensitivity by skin testing is of doubtful value.
Lidocaine Hydrochloride Oral Topical Solution, USP 2% (Viscous) contains a local anesthetic agent and is administered topically. Lidocaine Hydrochloride Oral Topical Solution, USP 2% (Viscous) contains lidocaine hydrochloride, which is chemically designated as acetamide, 2-(diethylamino)-N-(2,6-dimethylphenyl)-, monohydrochloride, monohydrate and has the following structural formula:
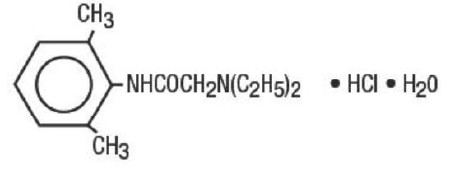
The molecular formula of lidocaine hydrochloride is C14H22N2O • HCl • H2O. The molecular weight is 288.81.
Lidocaine Hydrochloride Oral Topical Solution, USP 2% (Viscous) is a clear colorless viscous liquid with cherry flavor available as:
0121-0950-03: 100 mL polyethylene squeeze bottles.
0121-4950-15: 15 mL unit dose cup
0121-4950-40: Case contains 40 unit dose cups of 15 mL packaged in 4 trays of 10 unit dose cups each
The solutions should be stored at controlled room temperature 20° to 25°C (68° to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F). [See USP Controlled Room Temperature].