Livdelzi
(Seladelpar Lysine)Dosage & Administration
The recommended dosage of LIVDELZI is 10 mg orally once daily. Administer LIVDELZI with or without food. (
The recommended dosage of LIVDELZI is 10 mg orally once daily. Administer LIVDELZI with or without food
By using PrescriberAI, you agree to the AI Terms of Use.
Livdelzi Prescribing Information
LIVDELZI is indicated for the treatment of primary biliary cholangitis (PBC) in combination with ursodeoxycholic acid (UDCA) in adults who have had an inadequate response to UDCA, or as monotherapy in patients unable to tolerate UDCA.
This indication is approved under accelerated approval based on a reduction of alkaline phosphatase (ALP)
The efficacy of LIVDELZI was evaluated in Trial 1 (NCT04620733), a 12-month, randomized, double-blind, placebo-controlled trial. The study included 193 adult patients with PBC with an inadequate response or intolerance to UDCA. Patients were included in the trial if their ALP was greater than or equal to 1.67-times the ULN and total bilirubin (TB) was less than or equal to 2-times the ULN. Patients were excluded from the trial if they had other chronic liver diseases, clinically important hepatic decompensation including portal hypertension with complications, or cirrhosis with complications (e.g., Model for End Stage Liver Disease [MELD] score of 12 or greater, known esophageal varices or history of variceal bleeds, history of hepatorenal syndrome).
Patients were randomized to receive LIVDELZI 10 mg (N=128) or placebo (N=65) once daily for 12 months. LIVDELZI or placebo was administered in combination with UDCA in 181 (94%) patients during the trial, or as a monotherapy in 12 (6%) patients who were unable to tolerate UDCA.
The mean age of patients was 57 (Range: 28 to 75) years; 95% were female; 88% were White, 6% Asian, 2% Black or African American, and 3% American Indian or Alaska Native. Twenty-nine percent of the patients, 23% in the LIVDELZI 10 mg arm and 42% in the placebo arm, identified as Hispanic/Latino. Thirty-two percent of the patients, 38% in the LIVDELZI 10 mg arm and 20% in the placebo arm, were enrolled in the US.
At baseline, 18 (14%) of the LIVDELZI-treated patients and 9 (14%) of the placebo-treated patients met at least one of the following criteria: Fibroscan >16.9kPa; historical biopsy or radiological evidence suggestive of cirrhosis; platelet count < 140,000/µL with at least one additional laboratory finding including serum albumin < 3.5 g/dL, INR > 1.3, or TB > 1-time ULN; or clinical determination of cirrhosis by the investigator.
The mean baseline ALP concentration was 314 (Range: 161 to 786) units per liter (U/L), corresponding to 2.7-times ULN. The mean baseline TB concentration was 0.8 (Range: 0.3 to 1.9) mg/dL and was less than or equal to the ULN in 87% of the patients. Other mean baseline liver biochemistries were 48 (Range: 9 to 115) U/L for ALT and 40 (Range: 16 to 94) U/L for AST.
The primary endpoint was biochemical response at Month 12, where biochemical response was defined as achieving ALP less than 1.67-times ULN, an ALP decrease of greater than or equal to 15% from baseline, and TB less than or equal to ULN. ALP normalization (i.e., ALP less than or equal to ULN) at Month 12 was a key secondary endpoint. The ULN for ALP was defined as 116 U/L. The ULN for TB was defined as 1.1 mg/dL.
Table 3 presents results at Month 12 for the percentage of patients who achieved biochemical response, achieved each component of biochemical response, and achieved ALP normalization. LIVDELZI demonstrated greater improvement on biochemical response and ALP normalization at Month 12 compared to placebo. Overall, 87% of patients had a baseline of TB concentration less than or equal to ULN. Therefore, improvement in ALP was the main contributor to the biochemical response rate results at Month 12.
| LIVDELZI 10 mg Once Daily (N=128) | Placebo (N=65) | Treatment Difference % (95% CI)95% unstratified Miettinen and Nurminen confidence intervals (CIs) are provided. Patients who discontinued treatment prior to Month 12 or who had missing data were considered as non-responders. | |
|---|---|---|---|
Biochemical Response Rate, n (%),p<0.0001 for LIVDELZI 10 mg versus placebo. P-values were obtained using the Cochran–Mantel–Haenszel test stratified by baseline ALP level (<350 U/L versus ≥350 U/L) and baseline pruritus NRS (<4 versus ≥4). | 79 (62) | 13 (20) | 42 (28, 53) |
Components of Biochemical Response | |||
| ALP less than 1.67-times ULN, n (%) | 84 (66) | 17 (26) | 39 (25, 52) |
| Decrease in ALP of at least 15%, n (%) | 107 (84) | 21 (32) | 51 (37, 63) |
| TB less than or equal to ULN, n (%) | 104 (81) | 50 (77) | 4 (-7, 17) |
ALP Normalization, n (%)ALP normalization is defined as ALP less than or equal to ULN., | 32 (25) | 0 (0) | 25 (18, 33) |
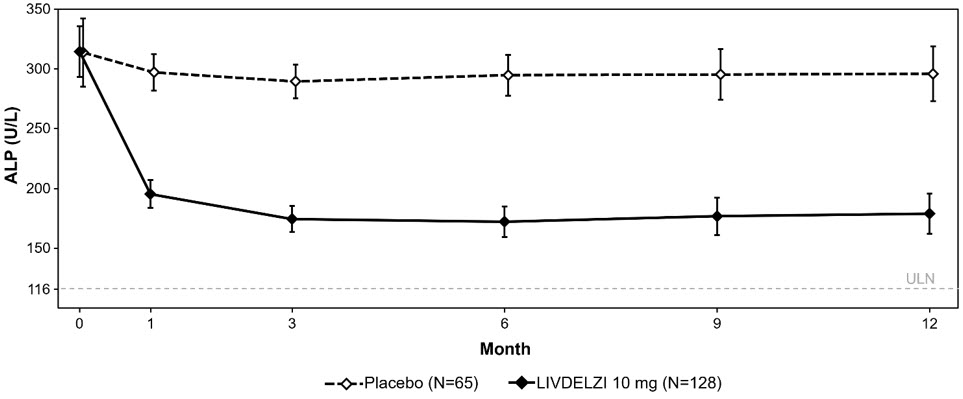
Figure 1 shows the mean (95% CI) levels of ALP over 12 months. There was a trend of lower ALP in LIVDELZI arm compared to placebo arm starting at Month 1 through Month 12.
Figure 1: MeanFigure 1 presents means and 95% Wald CIs for baseline, and least squares means and corresponding 95% CIs based on a mixed-effect model for repeated measures (MMRM) for Months 1, 3, 6, 9 and 12. The MMRM adjusts for baseline ALP, baseline ALP level (<350 U/L versus ≥350 U/L), baseline pruritus NRS (< 4 versus ≥4), time (in months), treatment arm, treatment-by-baseline ALP interaction, and treatment-by-time interaction. The least squares mean change from baseline in ALP at Month 12 was -134 (-151, -117) U/L and -17 (-40, 6) U/L in the LIVDELZI 10 mg and placebo arms, respectively.ALP in Adult Patients with PBC over 12 Months in Trial 1 |
 |
Biochemical response at Month 3 comparing LIVDELZI as a monotherapy to placebo was evaluated in a pooled analysis of a subset of patients from Trial 1 and another randomized, double-blind, placebo-controlled trial in a similar patient population. There was a trend of improvement on biochemical response at Month 3 in the LIVDELZI monotherapy group compared to the placebo group.

A single-item patient-reported outcome (PRO), the pruritus Numerical Rating Scale (NRS), evaluated patients' daily worst itching intensity on an 11-point rating scale with scores ranging from 0 ("no itching") to 10 ("worst itching imaginable") in Trial 1. The pruritus NRS was administered daily in a 14-day run-in period prior to randomization through Month 6.
Table 4 presents the results of the comparison between LIVDELZI and placebo on the key secondary endpoint evaluating the change from baseline in pruritus score at Month 6 in patients with baseline average pruritus scores greater than or equal to 4. The baseline average pruritus score for each patient was calculated by averaging the pruritus NRS scores administered in the run-in period and on Day 1 before treatment initiation. The pruritus scores at Month 6 for each patient were calculated by averaging the pruritus NRS scores within the last week in the month. Patients treated with LIVDELZI demonstrated greater improvement in pruritus compared with placebo.
| LIVDELZI 10 mg Once Daily (N=49) | Placebo (N=23) | |
|---|---|---|
Baseline Average Pruritus Score, Mean (SD) | 6.1 (1.4) | 6.6 (1.4) |
Change from Baseline in Pruritus Score at Month 6 | ||
| Mean (SE) | -3.2 (0.3) | -1.7 (0.4) |
| Mean difference vs. Placebo (95% CI) | -1.5 (-2.5, -0.5) p=0.0051 | |
The recommended dosage of LIVDELZI is 10 mg orally once daily. Administer LIVDELZI with or without food. (
The recommended dosage of LIVDELZI is 10 mg orally once daily. Administer LIVDELZI with or without food
Capsules: 10 mg, opaque, hard gelatin capsules, size 1, with light gray opaque body and a dark blue opaque cap, printed with "CBAY" on the cap and "10" on the body.
Hepatic Impairment: Monitor patients with cirrhosis for evidence of decompensation. Consider discontinuation if patient progresses to moderate or severe hepatic impairment (Child-Pugh B or C). (
No dosage adjustment is recommended for PBC patients with mild hepatic impairment (Child-Pugh A)
The safety and efficacy of LIVDELZI in patients with decompensated cirrhosis have not been established. Use of LIVDELZI is not recommended in patients who have or develop decompensated cirrhosis (e.g., ascites, variceal bleeding, hepatic encephalopathy).
Monitor patients with cirrhosis for evidence of decompensation. Consider discontinuing LIVDELZI if the patient progresses to moderate or severe hepatic impairment (Child-Pugh B or C).
None.
- Fractures: Consider the risk of fracture in patients treated with LIVDELZI. Monitor bone health according to current standards of care. ()
5.1 FracturesFractures occurred in 4% of LIVDELZI-treated patients compared to no placebo-treated patients
[see Adverse Reactions (6.1)].Consider the risk of fracture in the care of patients treated with LIVDELZI and monitor bone health according to current standards of care.
- Liver Test Abnormalities: Obtain baseline clinical and laboratory liver assessments prior to starting LIVDELZI and monitor during treatment. Interrupt or discontinue LIVDELZI if the liver tests worsen. ()
5.2 Liver Test AbnormalitiesLIVDELZI has been associated with dose-related increases in serum transaminase (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]) levels greater than 3-times upper limit of normal (ULN) in PBC patients receiving 50 mg once daily (5-times higher than the recommended dosage) and 200 mg (20-times higher than the recommended dosage) once daily. Transaminase levels returned to pretreatment levels upon LIVDELZI discontinuation. LIVDELZI 10 mg once daily did not show a similar pattern for increases in transaminase levels
[see Overdosage (10)].Obtain baseline clinical and laboratory assessments at treatment initiation with LIVDELZI and monitor thereafter according to routine patient management. Interrupt LIVDELZI treatment if the liver tests (ALT, AST, total bilirubin [TB], and/or alkaline phosphatase [ALP]) worsen, or the patient develops signs and symptoms consistent with clinical hepatitis (e.g., jaundice, right upper quadrant pain, eosinophilia). Consider permanent discontinuation if liver tests worsen after restarting LIVDELZI.
- Biliary Obstruction: Avoid use in patients with complete biliary obstruction. If biliary obstruction is suspected, interrupt LIVDELZI and treat as clinically indicated. ()
5.3 Biliary ObstructionAvoid use of LIVDELZI in patients with complete biliary obstruction. If biliary obstruction is suspected, interrupt LIVDELZI and treat as clinically indicated.