Loxapine - Loxapine capsule prescribing information
WARNING
Increased Mortality in Elderly Patients with Dementia-Related Psychosis Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Loxapine is not approved for the treatment of patients with dementia-related psychosis (see WARNINGS ).
INDICATIONS AND USAGE
Loxapine capsules are indicated for the treatment of schizophrenia. The efficacy of loxapine in schizophrenia was established in clinical studies which enrolled newly hospitalized and chronically hospitalized acutely ill schizophrenic patients as subjects.
DOSAGE AND ADMINISTRATION
Loxapine is administered, usually in divided doses, two to four times a day. Daily dosage (in terms of base equivalents) should be adjusted to the individual patient's needs as assessed by the severity of symptoms and previous history of response to antipsychotic drugs.
Oral Administration
Initial dosage of 10 mg twice daily is recommended, although in severely disturbed patients initial dosage up to a total of 50 mg daily may be desirable. Dosage should then be increased fairly rapidly over the first seven to ten days until there is effective control of symptoms of schizophrenia. The usual therapeutic and maintenance range is 60 mg to 100 mg daily. However, as with other drugs used to treat schizophrenia, some patients respond to lower dosage and others require higher dosage for optimal benefit. Daily dosage higher than 250 mg is not recommended.
Maintenance Therapy
For maintenance therapy, dosage should be reduced to the lowest level compatible with symptom control; many patients have been maintained satisfactorily at dosages in the range of 20 to 60 mg daily.
CONTRAINDICATIONS
Loxapine is contraindicated in comatose or severe drug-induced depressed states (alcohol, barbiturates, narcotics, etc.). Loxapine is contraindicated in individuals with known hypersensitivity to dibenzoxazepines.
ADVERSE REACTIONS
CNS Effects: Manifestations of adverse effects on the central nervous system, other than extrapyramidal effects, have been seen infrequently. Drowsiness, usually mild, may occur at the beginning of therapy or when dosage is increased. It usually subsides with continued loxapine therapy. The incidence of sedation has been less than that of certain aliphatic phenothiazines and slightly more than the piperazine phenothiazines. Dizziness, faintness, staggering gait, shuffling gait, muscle twitching, weakness, insomnia, agitation, tension, seizures, akinesia, slurred speech, numbness, and confusional states have been reported. Neuroleptic malignant syndrome (NMS) has been reported (see WARNINGS ). Extrapyramidal Symptoms - Neuromuscular (extrapyramidal) reactions during the administration of loxapine have been reported frequently, often during the first few days of treatment. In most patients, these reactions involved parkinsonian-like symptoms such as tremor, rigidity, excessive salivation, and masked facies. Akathisia (motor restlessness) also has been reported relatively frequently. These symptoms are usually not severe and can be controlled by reduction of loxapine dosage or by administration of antiparkinson drugs in usual dosage. Dystonia - Class Effect : Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency at higher doses of first generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger groups. Persistent Tardive Dyskinesia - As with all antipsychotic agents, tardive dyskinesia may appear in some patients on long-term therapy or may appear after drug therapy has been discontinued. The risk appears to be greater in elderly patients on high-dose therapy, especially females. The symptoms are persistent and in some patients appear to be irreversible. The syndrome is characterized by rhythmical involuntary movement of the tongue, face, mouth or jaw (e.g., protrusion of tongue, puffing of cheeks, puckering of mouth, chewing movements). Sometimes these may be accompanied by involuntary movements of extremities. There is no known effective treatment for tardive dyskinesia; antiparkinson agents usually do not alleviate the symptoms of this syndrome. It is suggested that all antipsychotic agents be discontinued if these symptoms appear. Should it be necessary to reinstitute treatment, or increase the dosage of the agent, or switch to a different antipsychotic agent, the syndrome may be masked. It has been suggested that fine vermicular movements of the tongue may be an early sign of the syndrome, and if the medication is stopped at that time the syndrome may not develop. Cardiovascular Effects: Tachycardia, hypotension, hypertension, orthostatic hypotension, lightheadedness, and syncope have been reported. A few cases of ECG changes similar to those seen with phenothiazines have been reported. It is not known whether these were related to loxapine administration. Hematologic: Rarely, agranulocytosis, thrombocytopenia, leukopenia. Skin: Dermatitis, edema (puffiness of face), pruritus, rash, alopecia, and seborrhea have been reported with loxapine. Anticholinergic Effects: Dry mouth, nasal congestion, constipation, blurred vision, urinary retention, and paralytic ileus have occurred. Gastrointestinal: Nausea and vomiting have been reported in some patients. Hepatocellular injury (i.e., SGOT/SGPT elevation) has been reported in association with loxapine administration and rarely, jaundice and/or hepatitis questionably related to loxapine treatment. Other Adverse Reactions: Weight gain, weight loss, dyspnea, ptosis, hyperpyrexia, flushed facies, headache, paresthesia, and polydipsia have been reported in some patients. Rarely, galactorrhea, amenorrhea, gynecomastia, and menstrual irregularity of uncertain etiology have been reported. To report SUSPECTED ADVERSE EVENTS, contact Elite Laboratories, Inc. at 1-888-852-6657 or FDA at 1-800-FDA-1088 or http://www.fda.gov/ for voluntary reporting of adverse reactions.
Drug Interactions
There have been rare reports of significant respiratory depression, stupor and/or hypotension with the concomitant use of loxapine and lorazepam. The risk of using loxapine in combination with CNS-active drugs has not been systematically evaluated. Therefore, caution is advised if the concomitant administration of loxapine and CNS-active drugs is required.
DESCRIPTION
Loxapine, a dibenzoxazepine compound, represents a subclass of tricyclic antipsychotic agents, chemically distinct from the thioxanthenes, butyrophenones, and phenothiazines. Chemically, it is 2-Chloro-11-(4-methyl-1-piperazinyl)dibenz[ b,f ][1,4]oxazepine. It is present as the succinate salt.
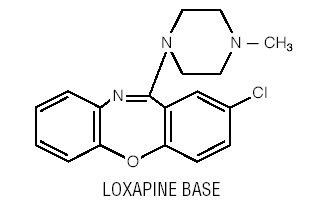
Each capsule for oral administration, contains loxapine succinate, USP 6.8 mg, 13.6 mg, 34.0 mg or 68.0 mg equivalent to 5 mg, 10 mg, 25 mg or 50 mg of loxapine base respectively. It also contains the following inactive ingredients: colloidal silicon dioxide, hypromellose, lactose monohydrate, magnesium stearate and titanium dioxide. The 5 mg capsule contains FD&C Blue #1 and FD&C Yellow #6; the 10 mg capsule contains FDA/E172 Yellow Iron Oxide, FD&C Blue #1, D&C Yellow #10 and FD&C Yellow #6; the 25 mg capsule contains FDA/E172 Yellow Iron Oxide, FD&C Blue #1 and D&C Yellow #10; the 50 mg capsule contains FDA/E172 Yellow Iron Oxide and FD&C Blue #1. The ink ingredients are common for all strengths: the capsule imprint ink contains shellac glaze, ferrosoferric oxide, butyl alcohol, propylene glycol, FD&C Blue #2 Aluminum Lake, FD&C Red #40 Aluminum Lake, D&C Yellow #10 Aluminum Lake, and FD&C Blue #1 Aluminum Lake.
CLINICAL PHARMACOLOGY
Pharmacodynamics
Pharmacologically, loxapine is an antipsychotic for which the exact mode of action has not been established. However, changes in the level of excitability of subcortical inhibitory areas have been observed in several animal species in association with such manifestations of tranquilization as calming effects and suppression of aggressive behavior. In normal human volunteers, signs of sedation were seen within 20 to 30 minutes after administration, were most pronounced within one and one-half to three hours, and lasted through 12 hours. Similar timing of primary pharmacologic effects was seen in animals.
Absorption, Distribution, Metabolism, and Excretion
Absorption of loxapine following oral or parenteral administration is virtually complete. The drug is removed rapidly from the plasma and distributed in tissues. Animal studies suggest an initial preferential distribution in lungs, brain, spleen, heart, and kidney. Loxapine is metabolized extensively and is excreted mainly in the first 24 hours. Metabolites are excreted in the urine in the form of conjugates and in the feces unconjugated.
HOW SUPPLIED
Loxapine Capsules, USP are available in the following strengths: Loxapine succinate, USP 6.8 mg equivalent to 5 mg loxapine, black ink, hard shell capsules Size 2, opaque, with dark green body and cap imprinted with " E525 " on cap and body, are supplied in bottles of 100 NDC# 64850-890-01. Loxapine succinate, USP 13.6 mg equivalent to 10 mg loxapine,black ink, hard shell capsules Size 2, opaque, yellow cap and dark green body imprinted with " E526 " on cap and body are supplied in bottles of 100 NDC# 64850-891-01. Loxapine succinate, USP 34.0 mg equivalent to 25 mg loxapine, black ink, hard shell capsules Size 2, opaque, with light green cap and dark green body imprinted with " E527 " on cap and body, are supplied in bottles of 100 NDC# 64850-892-01. Loxapine succinate, USP 68.0 mg equivalent to 50 mg loxapine,black ink, hard shell capsules Size 2, opaque, with light blue cap and dark green body imprinted with " E528 " on cap and body, are supplied in bottles of 100 NDC# 64850-893-01. Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Dispense in a tight container as defined in the USP, with a child-resistant closure, as required. Manufactured by: Elite Laboratories, Inc. Northvale, NJ 07647 USA IN0537 Revised 02/25