Mysoline
(Primidone)Mysoline Prescribing Information
MYSOLINE, used alone or concomitantly with other anticonvulsants, is indicated in the control of grand mal, psychomotor, and focal epileptic seizures. It may control grand mal seizures refractory to other anticonvulsant therapy.
Patients 8 years of age and older who have received no previous treatment may be started on MYSOLINE according to the following regimen using either 50 mg or scored 250 mg MYSOLINE tablets:
Days 1 to 3: 100 to 125 mg at bedtime.
Days 4 to 6: 100 to 125 mg twice a day.
Days 7 to 9: 100 to 125 mg three times a day.
Day 10 to maintenance: 250 mg three times a day.
For most adults and children 8 years of age and over, the usual maintenance dosage is three to four 250 mg MYSOLINE tablets in divided doses (250 mg three times a day or four times a day). If required, an increase to five or six 250 mg tablets daily may be made, but daily doses should not exceed 500 mg four times a day.
Dosage should be individualized to provide maximum benefit. In some cases, serum blood level determinations of primidone may be necessary for optimal dosage adjustment. The clinically effective serum level for primidone is between 5 to 12 mcg/mL.
INITIAL: ADULTS AND CHILDREN OVER 8 | ||||||
|---|---|---|---|---|---|---|
KEY: •=50 mg tablet; ●=250 mg tablet | ||||||
DAY | 1 | 2 | 3 | 4 | 5 | 6 |
AM | •• | •• | •• | |||
NOON | ||||||
PM | •• | •• | •• | •• | •• | •• |
DAY | 7 | 8 | 9 | 10 | 11 | 12 |
AM | •• | •• | •• | ● | Adjust to | |
NOON | •• | •• | •• | ● | ||
PM | •• | •• | •• | ● | ||
Primidone is contraindicated in: 1) patients with porphyria and 2) patients who are hypersensitive to phenobarbital (see
MYSOLINE raises electro- or chemoshock seizure thresholds or alters seizure patterns in experimental animals. The mechanism(s) of primidone’s antiepileptic action is not known.
Primidone per se has anticonvulsant activity as do its two metabolites, phenobarbital and phenylethylmalonamide (PEMA). In addition to its anticonvulsant activity, PEMA potentiates the anticonvulsant activity of phenobarbital in experimental animals.
The most frequently occurring early side effects are ataxia and vertigo. These tend to disappear with continued therapy, or with reduction of initial dosage. Occasionally, the following have been reported: nausea, anorexia, vomiting, fatigue, hyperirritability, emotional disturbances, sexual impotency, diplopia, nystagmus, drowsiness, and morbilliform skin eruptions. Granulocytopenia, agranulocytosis, and red-cell hypoplasia and aplasia, have been reported rarely. These and, occasionally, other persistent or severe side effects may necessitate withdrawal of the drug. Megaloblastic anemia may occur as a rare idiosyncrasy to MYSOLINE and to other anticonvulsants. The anemia responds to folic acid without necessity of discontinuing medication.
Chemical name: 5-ethyldihydro-5-phenyl-4,6 (1H, 5H)-pyrimidinedione. Structural formula:
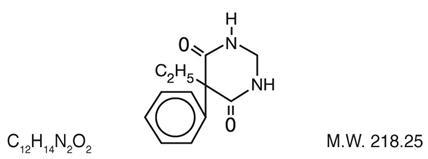
MYSOLINE (primidone) is a white, crystalline, highly stable substance, M.P. 279-284°C. It is poorly soluble in water (60 mg per 100 mL at 37°C) and in most organic solvents. It possesses no acidic properties, in contrast to its barbiturate analog.
MYSOLINE 50 mg and 250 mg tablets contain the following inactive ingredients: lactose
monohydrate, NF; magnesium stearate, NF; methylcellulose, USP; microcrystalline cellulose, NF;
purified water, USP; sodium lauryl sulfate, NF; sodium starch glycolate, NF; and talc, USP.
MYSOLINE 250 mg tablets also contain ferric oxide yellow, NF.
_______________________________________________________________________________
Modified square, flat faced, beveled edge, compressed light yellow color tablet. One face is debossed (impressed) with “MYSOLINE” and “250” that are divided by a debossed bisect line. The opposite side is embossed with the letter “M”, in bottles of 100 (NDC 66490-691-10).
Modified square, flat faced, beveled edge, compressed white color tablet. One face is debossed (impressed) with “MYSOLINE” and “50” that are divided by a debossed bisect line. The opposite side is embossed with the letter “M”, in bottles of 100 (NDC 66490-690-10).
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Bausch Health Companies Inc.
Steinbach, MB R5G 1Z7, Canada
MYSOLINE is a trademark of Bausch Health Companies Inc. or its affiliates.
© 2020 Bausch Health Companies Inc. or its affiliates
9648502 20002938