Nascobal patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
2.1 Testing and Other Considerations Prior to Dosing
Prior to treatment, obtain hematocrit, reticulocyte count, vitamin B 12 , folate, and iron levels [see Dosage and Administration (2.4) ] . Consider the potential for concomitant drugs to interfere with vitamin B 12 and folate diagnostic blood assays [see Drug Interactions (7) ] .
In patients with suspected cobalamin hypersensitivity, consider administering an intradermal test dose of parenteral vitamin B 12 prior to use of NASCOBAL [see Warnings and Precautions (5.2) ] .
Recommended Dosage
The recommended initial dose of NASCOBAL is one spray (500 mcg) administered in ONE nostril once weekly. Administer NASCOBAL at least one hour before or one hour after ingestion of hot foods or liquids since hot foods may cause nasal secretions and a resulting loss of medication. Defer use of NASCOBAL in patients with nasal congestion, allergic rhinitis, or upper respiratory infections until after symptoms have subsided.
Monitoring, Dosage Modifications, and Treatment Duration
Monitoring for Response and Safety
Monitor serum B 12 levels periodically during therapy to establish adequacy of therapy. Obtain a serum B 12 level and peripheral blood count one month after treatment initiation, then subsequently at intervals of 3 to 6 months [see Warnings and Precautions (5.3) ] .
Dosage Modifications
If serum levels of B 12 decline after one month of treatment with NASCOBAL, consider increasing the dose. Assess serum B 12 level one month after each dose adjustment. If serum B 12 levels are persistently low, consider alternative therapy (e.g., intramuscular or subcutaneous vitamin B 12 therapy).
Treatment Duration
In patients whose underlying cause of vitamin B 12 deficiency has been corrected and are deemed no longer at risk for vitamin B 12 deficiency, discontinue NASCOBAL. The safety and effectiveness of continued long-term use in these individuals has not been established.
In patients with pernicious anemia, continue appropriate vitamin B 12 treatment indefinitely.
2.4 Administration of NASCOBAL with Other Therapy
NASCOBAL should be administered with other therapy(ies) in:
- Patients with concurrent folate and vitamin B 12 deficiency:Administer folic acid in addition to NASCOBAL
- Patients with concurrent iron and vitamin B 12 deficiency:Administer iron in addition to NASCOBAL
- Patients with correctible causes of vitamin B 12 deficiency:Consider measures to treat the underlying condition associated with vitamin B 12 deficiency in addition to treatment with NASCOBAL

By using PrescriberAI, you agree to the AI Terms of Use.
Nascobal prescribing information
INDICATIONS AND USAGE
NASCOBAL is indicated for:
- Vitamin B 12 maintenance therapy in adult patients with pernicious anemia who are in remission following intramuscular vitamin B 12 therapy and who have no nervous system involvement
- Treatment of adult patients with dietary, drug-induced, or malabsorption-related vitamin B 12 deficiency not due to pernicious anemia
- Prevention of vitamin B 12 deficiency in adult patients with vitamin B 12 requirements in excess of normal
Limitations of Use
- NASCOBAL should not be used for the vitamin B 12 absorption test (Schilling test).
- In patients with correctible or temporary causes of vitamin B 12 deficiency, the benefit of continued long-term use of NASCOBAL following adequate correction of vitamin B 12 deficiency and underlying disease has not been established.
- The effectiveness of NASCOBAL in patients with active symptoms of nasal congestion, allergic rhinitis or upper respiratory infection has not been determined. Treatment with NASCOBAL should be deferred until symptoms have subsided.
DOSAGE AND ADMINISTRATION
2.1 Testing and Other Considerations Prior to Dosing
Prior to treatment, obtain hematocrit, reticulocyte count, vitamin B 12 , folate, and iron levels [see Dosage and Administration (2.4) ] . Consider the potential for concomitant drugs to interfere with vitamin B 12 and folate diagnostic blood assays [see Drug Interactions (7) ] .
In patients with suspected cobalamin hypersensitivity, consider administering an intradermal test dose of parenteral vitamin B 12 prior to use of NASCOBAL [see Warnings and Precautions (5.2) ] .
Recommended Dosage
The recommended initial dose of NASCOBAL is one spray (500 mcg) administered in ONE nostril once weekly. Administer NASCOBAL at least one hour before or one hour after ingestion of hot foods or liquids since hot foods may cause nasal secretions and a resulting loss of medication. Defer use of NASCOBAL in patients with nasal congestion, allergic rhinitis, or upper respiratory infections until after symptoms have subsided.
Monitoring, Dosage Modifications, and Treatment Duration
Monitoring for Response and Safety
Monitor serum B 12 levels periodically during therapy to establish adequacy of therapy. Obtain a serum B 12 level and peripheral blood count one month after treatment initiation, then subsequently at intervals of 3 to 6 months [see Warnings and Precautions (5.3) ] .
Dosage Modifications
If serum levels of B 12 decline after one month of treatment with NASCOBAL, consider increasing the dose. Assess serum B 12 level one month after each dose adjustment. If serum B 12 levels are persistently low, consider alternative therapy (e.g., intramuscular or subcutaneous vitamin B 12 therapy).
Treatment Duration
In patients whose underlying cause of vitamin B 12 deficiency has been corrected and are deemed no longer at risk for vitamin B 12 deficiency, discontinue NASCOBAL. The safety and effectiveness of continued long-term use in these individuals has not been established.
In patients with pernicious anemia, continue appropriate vitamin B 12 treatment indefinitely.
2.4 Administration of NASCOBAL with Other Therapy
NASCOBAL should be administered with other therapy(ies) in:
- Patients with concurrent folate and vitamin B 12 deficiency:Administer folic acid in addition to NASCOBAL
- Patients with concurrent iron and vitamin B 12 deficiency:Administer iron in addition to NASCOBAL
- Patients with correctible causes of vitamin B 12 deficiency:Consider measures to treat the underlying condition associated with vitamin B 12 deficiency in addition to treatment with NASCOBAL
DOSAGE FORMS AND STRENGTHS
Nasal spray: 500 mcg/0.1 mL (per actuation), packaged in a single-use device containing 0.125 mL of solution
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
The limited available data on NASCOBAL in pregnant women are insufficient to inform a drug-associated risk of adverse developmental outcomes. However, vitamin B12 is an essential vitamin and requirements are increased during pregnancy.
Animal reproduction studies have not been conducted with vitamin B 12 .
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Lactation
Risk Summary
Vitamin B 12 is present in the milk of lactating women in concentrations which approximate the mother’s vitamin B 12 blood level. Vitamin B 12 does not appear to pose more than a minimal risk to breastfeeding children.
Pediatric Use
Safety and effectiveness have not been established in pediatric patients.
Geriatric Use
Clinical studies of NASCOBAL did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
CONTRAINDICATIONS
NASCOBAL is contraindicated in patients with hypersensitivity to cobalt and/or vitamin B 12 or any of its excipients [see Warnings and Precautions (5.2) ] . Anaphylactic shock and death have been reported after parenteral vitamin B12 administration in sensitive patients.
WARNINGS AND PRECAUTIONS
Severe Optic Atrophy in Patients with Leber’s Disease
Patients with early Leber’s disease (hereditary optic nerve atrophy) who were treated with vitamin B 12 suffered severe and swift optic atrophy. Cyanocobalamin products, including NASCOBAL, is not recommended for use in patients with Leber’s optic atrophy. For patients with Leber’s disease requiring vitamin B 12 , consider alternative therapy (e.g., hydroxocobalamin) for B 12 supplementation.
5.2 Anaphylactic Reactions
Anaphylactic shock and death have been reported after parenteral vitamin B 12 administration. If patients are to start NASCOBAL before having tolerated cyanocobalamin parenterally, consider administering an intradermal test dose of parenteral vitamin B 12 to patients suspected of cyanocobalamin hypersensitivity [see Dosage and Administration (2.1) ] .
5.3 Masking of Folate Deficiency with Vitamin B12 Use
Doses of vitamin B 12 exceeding 10 mcg daily may produce hematologic response in patients with folate deficient megaloblastic anemia and may therefore mask a previously unrecognized folate deficiency. Vitamin B 12 is not a substitute for folic acid [see Dosage and Administration (2.4) ] . Assess both vitamin B 12 and folate levels prior to initiating therapy with vitamin B 12, including NASCOBAL, or with folic acid [see Dosage and Administration (2.1) ] .
Hypokalemia and Thrombocytosis Due to Intense Treatment of Megaloblastic Anemia
Hypokalemia and sudden death may occur in severe megaloblastic anemia that is treated intensely with vitamin B 12 . Hypokalemia and thrombocytosis can occur upon conversion of severe megaloblastic anemia to normal erythropoiesis with vitamin B 12 therapy. Therefore, serum potassium levels and platelet count should be monitored carefully during therapy [see Dosage and Administration (2.3) ] .
5.5 Unmasking of Polycythemia Vera
Vitamin B 12 deficiency may suppress the signs of polycythemia vera. Treatment with vitamin B 12 may unmask this condition. Patients exhibiting clinical or hematologic response consistent with polycythemia vera should be referred for further evaluation.
ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Severe Optic Atrophy in Patients with Leber’s Disease [see Warnings and Precautions (5.1) ] .
- Anaphylactic Reactions [see Warnings and Precautions (5.2) ] .
- Hypokalemia and Thrombocytosis Due to Intense Treatment of Megaloblastic Anemia [see Warnings and Precautions (5.4) ] .
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reactions described in Table 1 below are based on data from an eight week cross over trial in which vitamin B12 deficient patients in hematologic remission received one vitamin B12 intramuscular injection (N=25) and then received once weekly intranasal administration of another nasal cyanocobalamin formulation (N=24) for 4 weeks.
| Adverse Reaction | Number of Patients (%) | |
| Another Cyanocobalamin Nasal Formulation, 500 mcg (n=24) | Intramuscular Cyanocobalamin•, 100 mcg (n=25) | |
| Infection a | 3 (13) | 3 (12) |
| Headache | 1 (4) | 5 (20) |
| Asthenia | 1 (4) | 4 (16) |
| Nausea | 1 (4) | 1 (4) |
| Glossitis | 1 (4) | 0 (0) |
| Paresthesia | 1 (4) | 1 (4) |
| Rhinitis | 1 (4) | 2 (8) |
a Sore throat, common cold • The data are not an adequate basis for comparison of rates between the study drug and the active control | ||
DRUG INTERACTIONS
Chloramphenicol may decrease the efficacy of NASCOBAL when used for treatment of anemia. If NASCOBAL is used for the treatment of anemia concomitantly with chloramphenicol, monitor for reduced efficacy and if needed, consider an alternative therapy
DESCRIPTION
Cyanocobalamin is a synthetic form of vitamin B 12 . The chemical name is 5,6-dimethyl-benzimidazolyl cyanocobamide. The cobalt content is 4.35%. The molecular formula is C 63 H 88 CoN 14 O 14 P, which corresponds to a molecular weight of 1355.38 and the following structural formula:

Figure 1. Nascobal Chemical Structure
Cyanocobalamin occurs as dark red crystals or orthorhombic needles or crystalline red powder. It is very hygroscopic in the anhydrous form, and sparingly to moderately soluble in water (1:80). Its pharmacologic activity is destroyed by heavy metals (iron) and strong oxidizing or reducing agents (vitamin C), but not by autoclaving for short periods of time (15-20 minutes) at 121°C. The vitamin B 12 coenzymes are very unstable in light.
NASCOBAL (cyanocobalamin) nasal spray is a solution of cyanocobalamin, USP (vitamin B 12 ) for administration as a spray to the nasal mucosa. Each single-use device of NASCOBAL NASAL SPRAY contains 0.125 mL of a 500 mcg/0.1 mL solution of cyanocobalamin with, benzalkonium chloride in purified water, citric acid, glycerin and sodium citrate. The spray solution has a pH between 4.5 and 5.5. Each spray delivers an average of 500 mcg of cyanocobalamin per actuation.
CLINICAL PHARMACOLOGY
Mechanism of Action
Vitamin B 12 can be converted to coenzyme B 12 in tissues, and as such is essential for conversion of methylmalonate to succinate and synthesis of methionine from homocysteine, a reaction which also requires folate. In the absence of coenzyme B 12 , tetrahydrofolate cannot be regenerated from its inactive storage form, 5-methyltetrahydrofolate, and a functional folate deficiency occurs. Vitamin B 12 also may be involved in maintaining sulfhydryl (SH) groups in the reduced form required by many SH-activated enzyme systems. Through these reactions, vitamin B 12 is associated with fat and carbohydrate metabolism and protein synthesis.
Pharmacodynamics
Parenteral (intramuscular) administration of vitamin B 12 completely reverses the megaloblastic anemia and GI symptoms of vitamin B 12 deficiency; the degree of improvement in neurologic symptoms depends on the duration and severity of the lesions, although progression of the lesions is immediately arrested. In pernicious anemia patients, once weekly intranasal dosing with 500 mcg B 12 gel resulted in a consistent increase in pre-dose serum B 12 levels during one month of treatment (p < 0.003) above that seen one month after 100 mcg intramuscular dose (Figure 2).

Figure 2. Vitamin B 12 Serum Trough Levels After Intramuscular Solution (IM) of 100 mcg and Nasal Gel (IN) Administration of 500 mcg Cyanocobalamin After Weekly Doses.
Pharmacokinetics
Absorption
Vitamin B 12 is bound to intrinsic factor during transit through the stomach; separation occurs in the terminal ileum in the presence of calcium, and vitamin B 12 enters the mucosal cell for absorption. It is then transported by the transcobalamin binding proteins. A small amount (approximately 1% of the total amount ingested) is absorbed by simple diffusion, but this mechanism is adequate only with very large doses.
A three way crossover study in 25 fasting healthy subjects was conducted to compare the bioavailability of the B 12 nasal spray to the B 12 nasal gel and to evaluate the relative bioavailability of the nasal formulations as compared to the intramuscular injection. The peak concentrations after administration of intranasal spray were reached in 1.25 +/- 1.9 hours. The mean peak plasma concentration (C max ) of B 12 , obtained after baseline correction, following administration of intranasal spray were 748 +/-549 pg/mL. The bioavailability of the B 12 nasal spray was found to be 10% less than the B 12 nasal gel.
Distribution
In the blood, B 12 is bound to transcobalamin II, a specific B-globulin carrier protein, and is distributed and stored primarily in the liver and bone marrow.
Elimination
About 3-8 mcg of B 12 is secreted into the GI tract daily via the bile and undergoes some enterohepatic recycling; in normal subjects with sufficient intrinsic factor, all but about 1 mcg is reabsorbed. When B 12 is administered in doses which saturate the binding capacity of plasma proteins and the liver, the unbound B 12 is rapidly eliminated in the urine. Retention of B 12 in the body is dose-dependent. About 80-90% of an intramuscular dose up to 50 mcg is retained in the body; this percentage drops to 55% for a 100 mcg dose, and decreases to 15% when a 1000 mcg dose is given.
NONCLINICAL TOXICOLOGY
Carcinogenesis and Mutagenesis and Impairment of Fertility
Long-term studies in animals to evaluate carcinogenic potential have not been done.
HOW SUPPLIED/STORAGE AND HANDLING
NASCOBAL is a nasal spray available in a dosage strength of 500 mcg cyanocobalamin/0.1 mL (per actuation). It is supplied in boxes of 4 single-use nasal spray devices and a package insert (NDC 49884-270-82).
Protect from light. Keep covered in carton until ready to use. Store upright at controlled room temperature 15°C to 30°C (59°F to 86°F). Protect from freezing.
INSTRUCTIONS FOR USE
NASCOBAL (NAZ. co. bal)
(cyanocobalamin) nasal spray
NASCOBAL is for use in your nose only.
Read this Instructions for Use before you start using NASCOBAL (cyanocobalamin) nasal spray and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Your healthcare provider should teach you how to use NASCOBAL before you use it for the first time. If you have any questions or do not understand the instructions, talk with your healthcare provider or pharmacist.
NASCOBAL spray contains only 1 spray. You do not need to prime NASCOBAL before you use it.
If you are eating hot foods or liquids, use NASCOBAL at least one hour before or one hour afterwards because eating hot food or drinking hot liquids too soon before or after taking NASCOBAL can cause you to have a runny nose and can affect the amount of NASCOBAL you receive.
How to use NASCOBAL?
Step 1 . Blow your nose gently to clear both nostrils.
Step 2 . Hold NASCOBAL with your thumb on the bottom and your index (pointer) finger and middle finger on either side of the nozzle (see Figure A).

(Figure A)
Step 3 . Gently close 1 side of your nose (nostril) with your other index finger. Insert the nozzle into the open nostril about half an inch or as far as it feels comfortable and tilt your head slightly forward (see Figure B). Do not press the plunger yet.
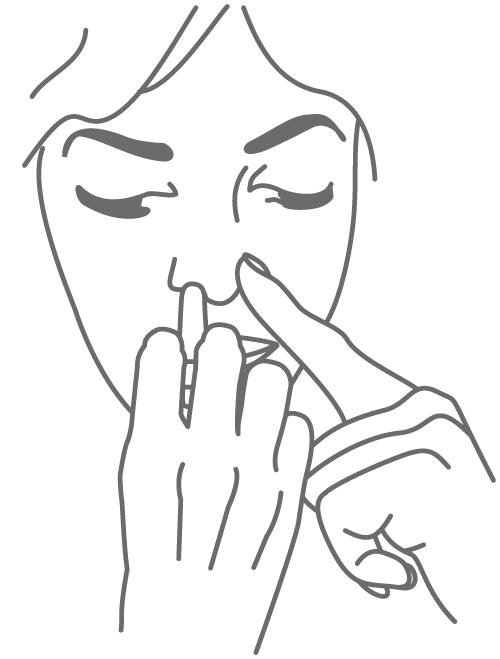
(Figure B)
Step 4 . Breathe in gently through your nose, close your mouth, and at the same time press the plunger firmly upwards with your thumb.
Step 5 . Remove the nozzle from your nostril. At the same time, keep your head straight for 10 to 20 seconds while gently breathing in through your nose and breathing out through your mouth.
How to dispose of (throw away) NASCOBAL?
NASCOBAL can be disposed of in your household trash. Place the NASCOBAL nasal spray in a container such as a zip-top or sealable plastic bag, and throw the container away in your household trash.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 11/2018
Mechanism of Action
Vitamin B 12 can be converted to coenzyme B 12 in tissues, and as such is essential for conversion of methylmalonate to succinate and synthesis of methionine from homocysteine, a reaction which also requires folate. In the absence of coenzyme B 12 , tetrahydrofolate cannot be regenerated from its inactive storage form, 5-methyltetrahydrofolate, and a functional folate deficiency occurs. Vitamin B 12 also may be involved in maintaining sulfhydryl (SH) groups in the reduced form required by many SH-activated enzyme systems. Through these reactions, vitamin B 12 is associated with fat and carbohydrate metabolism and protein synthesis.