Nplate (Romiplostim)
Dosage & administration
By using PrescriberAI, you agree to the AI Terms of Use.
Nplate prescribing information
Warnings and Precautions, Thrombotic/Thromboembolic Complications (Thrombotic/thromboembolic complications have resulted from increases in platelet counts with Nplate use secondary to drug-induced thrombocytosis, regardless of the underlying disease. Thrombotic/ thromboembolic events including deep vein thrombosis (1.4%), pulmonary embolism (1.2%) and myocardial infarction (0.8%) have been observed with the use of Nplate in the ITP population. Other thrombotic events including transient ischemic attack have been reported. These events have occurred regardless of platelet counts. Portal vein thrombosis has been reported in patients with and without chronic liver disease receiving Nplate. In patients with ITP, to minimize the risk for thrombotic/thromboembolic complications, do not use Nplate in an attempt to normalize platelet counts. Follow the dose adjustment guidelines [see Dosage and Administration ( 2.1 )] .In the absence of myelosuppression induced by acute exposure to radiation, Nplate administration might cause excessive increases in platelet counts and may cause thrombotic and thromboembolic complications [see Clinical Pharmacology (12.2)]. | 02/2025 |
Warnings and Precautions, Loss of Response to Nplate (Hyporesponsiveness or failure to maintain a platelet response with Nplate may occur due to neutralizing antibodies or other causes [see Adverse Reactions ( 6.3 )] . Discontinue Nplate if the platelet count does not increase to a level sufficient to avoid clinically important bleeding after 4 weeks at the highest weekly dose of 10 mcg/kg. | 02/2025 |
Nplate is a thrombopoietin receptor agonist indicated for the treatment of thrombocytopenia in:
- Adult patients with immune thrombocytopenia (ITP) who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy. ()
1.1 Patients with Immune Thrombocytopenia (ITP)Nplate is indicated for the treatment of thrombocytopenia in:
- Adult patients with immune thrombocytopenia (ITP) who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
- Pediatric patients 1 year of age and older with ITP for at least 6 months who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
- Pediatric patients 1 year of age and older with ITP for at least 6 months who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy. ()
1.1 Patients with Immune Thrombocytopenia (ITP)Nplate is indicated for the treatment of thrombocytopenia in:
- Adult patients with immune thrombocytopenia (ITP) who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
- Pediatric patients 1 year of age and older with ITP for at least 6 months who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
Nplate is indicated to increase survival in adults and in pediatric patients (including term neonates) acutely exposed to myelosuppressive doses of radiation (Hematopoietic Syndrome of Acute Radiation Syndrome [HS-ARS]). (
Nplate is indicated to increase survival in adults and in pediatric patients (including term neonates) acutely exposed to myelosuppressive doses of radiation
- Nplate is not indicated for the treatment of thrombocytopenia due to myelodysplastic syndrome (MDS) or any cause of thrombocytopenia other than ITP[see Warnings and Precautions (5.1)].
- Nplate should be used only in patients with ITP whose degree of thrombocytopenia and clinical condition increases the risk for bleeding.
- Nplate should not be used in an attempt to normalize platelet counts[see Warnings and Precautions (5.2)].
- Nplate is not indicated for the treatment of thrombocytopenia due to myelodysplastic syndrome (MDS) or any cause of thrombocytopenia other than ITP.
- Nplate should be used only in patients with ITP whose degree of thrombocytopenia and clinical condition increases the risk for bleeding.
- Nplate should not be used in an attempt to normalize platelet counts. ()1INDICATIONSAND USAGE
Nplate is a thrombopoietin receptor agonist indicated for the treatment of thrombocytopenia in:
- Adult patients with immune thrombocytopenia (ITP) who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
- Pediatric patients 1 year of age and older with ITP for at least 6 months who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
Nplate is indicated to increase survival in adults and in pediatric patients (including term neonates) acutely exposed to myelosuppressive doses of radiation (Hematopoietic Syndrome of Acute Radiation Syndrome [HS-ARS]).
Limitations of Use:- Nplate is not indicated for the treatment of thrombocytopenia due to myelodysplastic syndrome (MDS) or any cause of thrombocytopenia other than ITP.
- Nplate should be used only in patients with ITP whose degree of thrombocytopenia and clinical condition increases the risk for bleeding.
- Nplate should not be used in an attempt to normalize platelet counts.
1.1 Patients with Immune Thrombocytopenia (ITP)Nplate is indicated for the treatment of thrombocytopenia in:
- Adult patients with immune thrombocytopenia (ITP) who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
- Pediatric patients 1 year of age and older with ITP for at least 6 months who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
1.2 Patients with Hematopoietic Syndrome of Acute Radiation SyndromeNplate is indicated to increase survival in adults and in pediatric patients (including term neonates) acutely exposed to myelosuppressive doses of radiation
[see Clinical Studies (14.3)].Limitations of Use:- Nplate is not indicated for the treatment of thrombocytopenia due to myelodysplastic syndrome (MDS) or any cause of thrombocytopenia other than ITP[see Warnings and Precautions (5.1)].
- Nplate should be used only in patients with ITP whose degree of thrombocytopenia and clinical condition increases the risk for bleeding.
- Nplate should not be used in an attempt to normalize platelet counts[see Warnings and Precautions (5.2)].
- Patients with Immune Thrombocytopenia (ITP)
- Recommended Initial Dose: 1 mcg/kg once weekly as a subcutaneous injection. Adjust dose based on platelet response. ()
2.1 Patients with Immune Thrombocytopenia (ITP)Use the lowest dose of Nplate to achieve and maintain a platelet count ≥ 50 × 109/L as necessary to reduce the risk for bleeding. Administer Nplate as a weekly subcutaneous injection with dose adjustments based upon the platelet count response.
The prescribed Nplate dose may consist of a very small volume (e.g., 0.15 mL). Administer Nplate only with a syringe that contains 0.01 mL graduations.
Discontinue Nplate if the platelet count does not increase to a level sufficient to avoid clinically important bleeding after 4 weeks of Nplate therapy at the maximum weekly dose of 10 mcg/kg
[see Warnings and Precautions (5.3)].Obtain complete blood counts (CBCs), including platelet counts, weekly during the dose adjustment phase of Nplate therapy and then monthly following establishment of a stable Nplate dose. Obtain CBCs, including platelet counts, weekly for at least 2 weeks following discontinuation of Nplate.
For Adult Patients with ITPThe initial dose of Nplate is 1 mcg/kg. Actual body weight at initiation of treatment should always be used when calculating the initial dose. In adults, future dose adjustments are based on changes in platelet counts only.
Adjust the weekly dose of Nplate by increments of 1 mcg/kg until the patient achieves a platelet count ≥ 50 × 109/L as necessary to reduce the risk for bleeding; do not exceed a maximum weekly dose of 10 mcg/kg. In clinical studies, most adult patients who responded to Nplate achieved and maintained platelet counts ≥ 50 × 109/L with a median dose of 2-3 mcg/kg.
Adjust the dose as follows for adult patients:
- If the platelet count is < 50 × 109/L, increase the dose by 1 mcg/kg.
- If platelet count is > 200 × 109/L and ≤ 400 × 109/L for 2 consecutive weeks, reduce the dose by 1 mcg/kg.
- If platelet count is > 400 × 109/L, do not dose. Continue to assess the platelet count weekly. After the platelet count has fallen to < 200 × 109/L, resume Nplate at a dose reduced by 1 mcg/kg.
For Pediatric Patients with ITPThe initial dose of Nplate is 1 mcg/kg. Actual body weight at initiation of treatment should always be used when calculating initial dose. In pediatric patients, future dose adjustments are based on changes in platelet counts and changes in body weight. Reassessment of body weight is recommended every 12 weeks.
Adjust the weekly dose of Nplate by increments of 1 mcg/kg until the patient achieves a platelet count ≥ 50 × 109/L as necessary to reduce the risk for bleeding; do not exceed a maximum weekly dose of 10 mcg/kg. In a pediatric placebo-controlled clinical study, the median of the most frequent dose of Nplate received by patients during weeks 17 through 24 was 5.5 mcg/kg.
Adjust the dose as follows for pediatric patients:
- If the platelet count is < 50 × 109/L, increase the dose by 1 mcg/kg.
- If platelet count is > 200 × 109/L and ≤ 400 × 109/L for 2 consecutive weeks, reduce the dose by 1 mcg/kg.
- If platelet count is > 400 × 109/L, do not dose. Continue to assess the platelet count weekly. After the platelet count has fallen to < 200 × 109/L, resume Nplate at a dose reduced by 1 mcg/kg.
- Recommended Initial Dose: 1 mcg/kg once weekly as a subcutaneous injection. Adjust dose based on platelet response. (
- Patients acutely exposed to myelosuppressive doses of radiation
- Recommended Dose: 10 mcg/kg administered once as a subcutaneous injection. Administer the dose as soon as possible after suspected or confirmed exposure to myelosuppressive doses of radiation. ()
2.2 Patients with Hematopoietic Syndrome of Acute Radiation SyndromeFor Adult and Pediatric Patients (including term neonates)The recommended dose of Nplate is 10 mcg/kg administered once as a subcutaneous injection. Administer the dose as soon as possible after suspected or confirmed exposure to radiation levels greater than 2 gray (Gy).
Administer Nplate regardless of whether a complete blood count (CBC) can be obtained. Estimate a patient's absorbed whole body radiation dose (i.e., level of radiation exposure) based on information from public health authorities, biodosimetry if available, or clinical findings such as time to onset of vomiting or lymphocyte depletion kinetics.
- Recommended Dose: 10 mcg/kg administered once as a subcutaneous injection. Administer the dose as soon as possible after suspected or confirmed exposure to myelosuppressive doses of radiation. (
- See Full Prescribing Information for instructions on reconstitution, preparation, and administration. ()
2.3 Preparation and AdministrationTo mitigate against medication errors (both overdose and underdose), ensure that these preparation and administration instructions are followed. Use aseptic technique. Only administer subcutaneously
[see Overdosage(10)].Nplate is supplied in single-dose vials as a sterile, preservative-free, white lyophilized powder that must be reconstituted as outlined in Table 1 and administered using a syringe with 0.01 mL graduations.
Calculation of Patient DoseMultiply the patient’s weight (kg) by the prescribed dose to obtain the Calculated Patient Dose.
Calculated Patient Dose (mcg) = Weight (kg) × Prescribed dose (mcg/kg) Reconstitutionand Dilutionof Nplate Single-DoseVials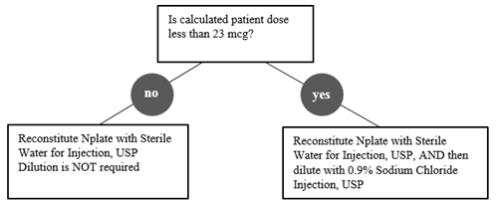
Reconstitute Nplate with Sterile Water for Injection, USP.
Do notreconstitute or dilute with Bacteriostatic Water for Injection, USP or dilute with Bacteriostatic Sodium Chloride Injection, USP. If the Calculated Patient Dose is less than 23 mcg, dilution with 0.9% Sodium Chloride Injection, USP is required to reduce the concentration of Nplate (see Table 1). This reduced concentration allows for low-doses to be accurately calculated, and consistently measured with a 0.01 mL graduated syringe.Table 1. Reconstitution and Dilution of Nplate Single-Dose Vials Calculated Patient Dose StrengthVial contains overfill to ensure delivery of labeled vial strength. Reconstitute with Sterile WaterAdd Sterile Water for Injection, USP directly to the vial. Dilute with Normal SalineAdd 0.9% Sodium Chloride Injection, USP directly to the vial. Final Concentration Calculated Dose greater than or equal to 23 mcg125 mcg 0.44 mL Not Required 500 mcg/mL 250 mcg 0.72 mL Not Required 500 mcg 1.2 mL Not Required Calculated Dose less than 23 mcg125 mcg 0.44 mL 1.38 mL 125 mcg/mL 250 mcg 0.72 mL 2.25 mL 500 mcg 1.2 mL 3.75 mL Gently swirl and invert the vial to reconstitute. Avoid excess or vigorous agitation:
DO NOT SHAKE. Generally, dissolution of Nplate takes less than 2 minutes. The reconstituted Nplate solution should be clear and colorless. Visually inspect the reconstituted solution for particulate matter and/or discoloration. Do not administer Nplate if particulate matter and/or discoloration is observed.Calculate Volume to Administer by dividing the Calculated Patient Dose (mcg) by the final concentration of prepared solution. See Table 2 for final concentrations.
Table 2. Administration of Prepared Nplate Solution Calculated Patient DoseFinal ConcentrationVolume to Administer (mL)Calculated Dose greater than or equal to 23mcg500 mcg/mL = Calculated Patient Dose / 500 mcg/mL Calculated Dose less than 23mcg125 mcg/mL = Calculated Patient Dose / 125 mcg/mL Administration of Prepared Nplate SolutionAdminister Nplate only using a syringe with 0.01 mL graduations for accurate dosage. Round volume to the nearest hundredth mL. Verify that the syringe contains the correct dosage.
Discard any unused portion.
Do notpool unused portions from the vials.Do notadminister more than one dose from a vial.Storage of Reconstituted SolutionReconstituted product with Sterile Water for Injection, USP that has not been further diluted can remain in the original vial at room temperature 25°C (77°F) or be refrigerated at 2°C to 8°C (36°F to 46°F) for up to 24 hours following reconstitution. Reconstituted product with Sterile Water for Injection, USP may be held in a syringe at room temperature 25°C (77°F) for a maximum of 4 hours following reconstitution. Protect product from light. Do not shake.
Storage of Diluted solution (after initial reconstitution)Reconstituted and further diluted product with 0.9% Sodium Chloride Injection, USP can be held in a syringe at room temperature 25°C (77°F) or in the original vial refrigerated at 2°C to 8°C (36°F to 46°F) for no longer than 4 hours prior to administration. Protect product from light. Do not shake.
For injection: 125 mcg, 250 mcg or 500 mcg of Nplate as a sterile, lyophilized, solid white powder in single-dose vials.
None.
- In some patients with MDS, Nplate increases blast cell counts and increases the risk of progression to acute myelogenous leukemia. ()
5.1 Risk of Progression of Myelodysplastic Syndromes to Acute Myelogenous LeukemiaProgression from myelodysplastic syndromes (MDS) to acute myelogenous leukemia (AML) has been observed in adult clinical trials with Nplate.
A randomized, double-blind, placebo-controlled trial enrolling adult patients with severe thrombocytopenia and International Prognostic Scoring System (IPSS) low or intermediate-1 risk MDS was terminated due to more cases of AML observed in the Nplate arm. This trial consisted of a 58-week study period with a 5-year long-term follow-up phase. The patients were randomized 2:1 to treatment with Nplate or placebo (167 Nplate, 83 placebo). During the 58-week study period, progression to AML occurred in 10 (6.0%) patients in the Nplate arm and 4 (4.8%) patients in the placebo arm (hazard ratio [95%CI] = 1.20 [0.38, 3.84]). Of the 250 patients, 210 (84.0%) entered the long-term follow-up phase of this study. With 5 years of follow-up, 29 (11.6%) patients showed progression to AML, including 20/168 (11.9%) patients in the Nplate arm versus 9/82 (11.0%) patients in the placebo arm (HR [95% CI] = 1.06 [0.48, 2.33]). The incidence of death (overall survival) was 55.7% (93/167) in the Nplate arm versus 54.2% (45/83) in the placebo arm (HR [95% CI] = 1.03 [0.72, 1.47]). In the baseline low IPSS group, there was a higher incidence of death in the Nplate arm [41.3% (19/46)] compared to the placebo arm [30.4% (7/23)] (HR [95% CI] = 1.59 [0.67, 3.80]).
In a single-arm trial of Nplate given to 72 patients with thrombocytopenia-related MDS, 8 (11.1%) patients were reported as having possible disease progression, of which 3 (4.2%) had confirmation of AML during follow-up. In addition, in 3 (4.2%) patients, increased peripheral blood blast cell counts decreased to baseline after discontinuation of Nplate.
Nplate is not indicated for the treatment of thrombocytopenia due to MDS or any cause of thrombocytopenia other than ITP.
- Thrombotic/thromboembolic complications have resulted from increases in platelet counts with Nplate use. Portal vein thrombosis has been reported in patients with chronic liver disease receiving Nplate. ()
5.2 Thrombotic/Thromboembolic ComplicationsThrombotic/thromboembolic complications have resulted from increases in platelet counts with Nplate use secondary to drug-induced thrombocytosis, regardless of the underlying disease. Thrombotic/ thromboembolic events including deep vein thrombosis (1.4%), pulmonary embolism (1.2%) and myocardial infarction (0.8%) have been observed with the use of Nplate in the ITP population. Other thrombotic events including transient ischemic attack have been reported. These events have occurred regardless of platelet counts. Portal vein thrombosis has been reported in patients with and without chronic liver disease receiving Nplate.In patients with ITP, to minimize the risk for thrombotic/thromboembolic complications, do not use Nplate in an attempt to normalize platelet counts. Follow the dose adjustment guidelines[see Dosage and Administration (2.1)].In the absence of myelosuppression induced by acute exposure to radiation, Nplate administration might cause excessive increases in platelet counts and may cause thrombotic and thromboembolic complications[see Clinical Pharmacology (12.2)]. - Severe thrombocytopenia may persist during Nplate treatment due to neutralizing antibodies or other causes. ()
5.3 Loss of Response to NplateHyporesponsiveness or failure to maintain a platelet response with Nplate may occur due to neutralizing antibodies or other causes[see Adverse Reactions (6.3)]. Discontinue Nplate if the platelet count does not increase to a level sufficient to avoid clinically important bleeding after 4 weeks at the highest weekly dose of 10 mcg/kg.