Orenitram
(Treprostinil)Dosage & Administration
By using PrescriberAI, you agree to the AI Terms of Use.
Orenitram Prescribing Information
Dosage and Administration (Take Orenitram with food. Swallow Orenitram tablets whole; do not crush, split, or chew. The recommended starting dose of Orenitram is 0.125 mg three times daily (TID) with food, taken approximately 8 hours apart or 0.25 mg twice daily (BID) with food, taken approximately 12 hours apart. Titrate by 0.125 mg TID or 0.25 or 0.5 mg BID not more frequently than every 3 to 4 days. Increase the dose to the highest tolerated dose. The recommended maximum daily dose is 120 mg. If dose increments are not tolerated, consider titrating slower. If intolerable pharmacologic effects occur, decrease the dose in increments of 0.125 mg TID or 0.25 mg BID. Avoid abrupt discontinuation [see Warnings and Precautions (5.1)] . | 08/2023 |
Orenitram is a prostacyclin mimetic indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1):
- To delay disease progression and to improve exercise capacity. The studies that established effectiveness included predominately patients with WHO functional class II-III symptoms and etiologies of idiopathic or heritable PAH (66%) or PAH associated with connective tissue disease (26%). ()
1.1 Pulmonary Arterial HypertensionOrenitram is indicated for the treatment of pulmonary arterial hypertension (PAH) (WHO Group 1) to delay disease progression and to improve exercise capacity.
The studies that established effectiveness included predominately patients with WHO functional class II-III symptoms and etiologies of idiopathic or heritable PAH (66%) or PAH associated with connective tissue disease (26%).
- Give with food. Swallow tablets whole; use only intact tablets. ()
2.1 Recommended DosingTake Orenitram with food. Swallow Orenitram tablets whole; do not crush, split, or chew.
The recommended starting dose of Orenitram is 0.125 mg three times daily (TID) with food, taken approximately 8 hours apart or 0.25 mg twice daily (BID) with food, taken approximately 12 hours apart.
Titrate by 0.125 mg TID or 0.25 or 0.5 mg BID not more frequently than every 3 to 4 days. Increase the dose to the highest tolerated dose. The recommended maximum daily dose is 120 mg.If dose increments are not tolerated, consider titrating slower. If intolerable pharmacologic effects occur, decrease the dose in increments of 0.125 mg TID or 0.25 mg BID.
Avoid abrupt discontinuation[see Warnings and Precautions (5.1)]. - Starting dose: 0.125 mg TID or 0.25 mg BID. ()
2.1 Recommended DosingTake Orenitram with food. Swallow Orenitram tablets whole; do not crush, split, or chew.
The recommended starting dose of Orenitram is 0.125 mg three times daily (TID) with food, taken approximately 8 hours apart or 0.25 mg twice daily (BID) with food, taken approximately 12 hours apart.
Titrate by 0.125 mg TID or 0.25 or 0.5 mg BID not more frequently than every 3 to 4 days. Increase the dose to the highest tolerated dose. The recommended maximum daily dose is 120 mg.If dose increments are not tolerated, consider titrating slower. If intolerable pharmacologic effects occur, decrease the dose in increments of 0.125 mg TID or 0.25 mg BID.
Avoid abrupt discontinuation[see Warnings and Precautions (5.1)]. - Titrate by 0.125 mg TID or by 0.25 mg or 0.5 mg BID, not more frequently than every 3 to 4 days as tolerated. The maximum daily dose is 120 mg. ()
2.1 Recommended DosingTake Orenitram with food. Swallow Orenitram tablets whole; do not crush, split, or chew.
The recommended starting dose of Orenitram is 0.125 mg three times daily (TID) with food, taken approximately 8 hours apart or 0.25 mg twice daily (BID) with food, taken approximately 12 hours apart.
Titrate by 0.125 mg TID or 0.25 or 0.5 mg BID not more frequently than every 3 to 4 days. Increase the dose to the highest tolerated dose. The recommended maximum daily dose is 120 mg.If dose increments are not tolerated, consider titrating slower. If intolerable pharmacologic effects occur, decrease the dose in increments of 0.125 mg TID or 0.25 mg BID.
Avoid abrupt discontinuation[see Warnings and Precautions (5.1)]. - If transitioning from intravenous (IV) or subcutaneous (SC) Remodulin®, the Orenitram dose should be increased while simultaneously decreasing the IV/SC infusion rate. ()
2.2 Transitioning from Subcutaneous or Intravenous Routes of Administration of TreprostinilDecrease the dose of Remodulin while simultaneously increasing the dose of Orenitram. The dose of Remodulin can be reduced up to 30 ng/kg/min per day and the dose of Orenitram simultaneously increased up to 6 mg per day (2 mg TID) if tolerated. The following equation can be used to estimate a target total daily dose of Orenitram in mg using a patient's dose of intravenous (IV)/subcutaneous (SC) treprostinil (in ng/kg/min) and weight (in kg).
Orenitram total daily dose (mg) = 0.0072 × Remodulin dose (ng/kg/min) × weight (kg) - Mild hepatic impairment (Child Pugh Class A): Initiate at 0.125 mg BID. Increment at 0.125 mg BID not more frequently than every 3 to 4 days. ()
2.3 Dose Adjustment in Patients with Hepatic ImpairmentIn patients with mild hepatic impairment (Child Pugh Class A) start at 0.125 mg BID with 0.125 mg BID dose increments not more frequently than every 3 to 4 days. Avoid use of Orenitram in patients with moderate hepatic impairment (Child Pugh Class B). Orenitram is contraindicated in patients with severe hepatic impairment (Child Pugh Class C) due to increases in systemic exposure
[see Contraindications (4), Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)]. - Avoid use in patients with moderate hepatic impairment. ()
2.3 Dose Adjustment in Patients with Hepatic ImpairmentIn patients with mild hepatic impairment (Child Pugh Class A) start at 0.125 mg BID with 0.125 mg BID dose increments not more frequently than every 3 to 4 days. Avoid use of Orenitram in patients with moderate hepatic impairment (Child Pugh Class B). Orenitram is contraindicated in patients with severe hepatic impairment (Child Pugh Class C) due to increases in systemic exposure
[see Contraindications (4), Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)].
Orenitram (treprostinil) extended-release tablets are available in the following five strengths:
- 0.125 mg [White tablet imprinted with UT 0.125]- 0.25 mg [Green tablet imprinted with UT 0.25]- 1 mg [Yellow tablet imprinted with UT 1]- 2.5 mg [Pink tablet imprinted with UT 2.5]- 5 mg [Red tablet imprinted with UT 5]
Limited published data from case reports with Orenitram use in pregnant women are not sufficient to assess for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. There are risks to the mother and the fetus associated with pulmonary arterial hypertension
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Severe hepatic impairment (Child Pugh Class C)
There is a marked increase in the systemic exposure to treprostinil in hepatically impaired patients
In patients with PAH, pharmacokinetics of treprostinil is dose-proportional for systemic exposure (AUC0-t) over the dose range from 0.5 to 15 mg BID. Upon repeat administration with a BID regimen, the accumulation in the systemic exposures to treprostinil is minimal and results in a peak-to-trough ratio of approximately 7. However, a TID regimen will reduce the peak-to-trough fluctuations to approximately 2.5 for the same total daily dose.
The absolute oral bioavailability of Orenitram is approximately 17%. Maximum treprostinil concentrations occur between approximately 4 and 6 hours following Orenitram administration. Time to reach steady-state concentrations for both BID and TID regimens is approximately 1 to 2 days.
The absorption of Orenitram is affected by food. The AUCinfof treprostinil was increased by 49% and the Cmaxwas increased by an average of 13% when Orenitram was administered following a high-fat, high-calorie meal compared to fasting conditions in healthy volunteers. The relative bioavailability of treprostinil following oral administration of Orenitram 1 mg is not significantly altered by meal types ranging from 250 to 500 calories in healthy volunteers.
When Orenitram 1 mg was administered with alcohol at 0.5 mg/kg or the equivalent of 3 servings (at the same time, or ± 1 hour relative to alcohol consumption), there was no significant change (10% to 20% increase) in the exposure to treprostinil compared to Orenitram administered alone.
The treprostinil component of Orenitram is highly bound to human plasma proteins, approximately 96% over a treprostinil concentration range of 0.01 to 10 µg/mL.
In a study conducted in healthy volunteers using [14C] treprostinil, treprostinil was extensively metabolized on the side chain of the molecule via oxidation, oxidative cleavage, dehydration, and glucuronic acid conjugation. Treprostinil is primarily metabolized by CYP2C8 and to a lesser extent by CYP2C9. No new major metabolites are found upon oral administration compared to parenteral administration of treprostinil. Only 1.13% and 0.19% is excreted as unchanged parent drug in the feces and urine, respectively. Based on
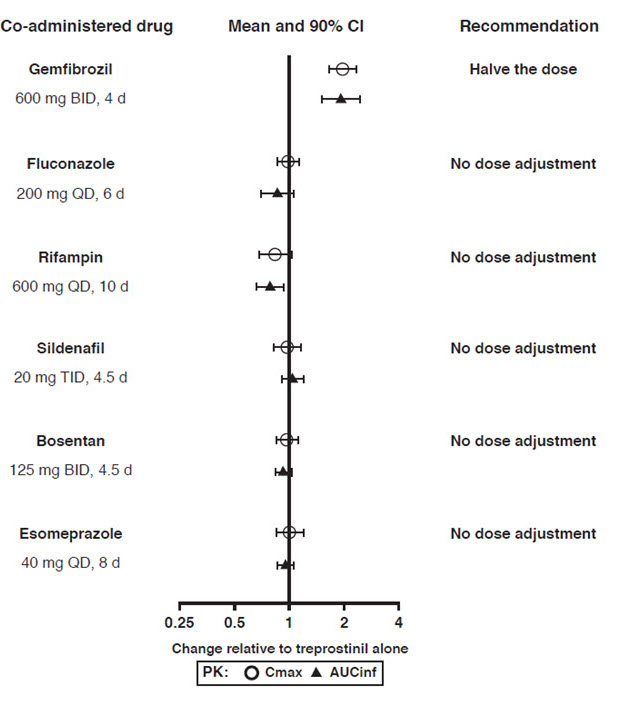
Results of drug interaction studies are shown in Figure 1. Only for the strong CYP2C8 inhibitor does the interaction affect dosing