Oxacillin
Oxacillin Prescribing Information
Oxacillin is indicated in the treatment of infections caused by penicillinase producing staphylococci which have demonstrated susceptibility to the drug. Cultures and susceptibility tests should be performed initially to determine the causative organism and its susceptibility to the drug (See
Intravenous administration provides peak serum levels approximately 5 minutes after the injection is completed. Slow I.V administration of 500 mg gives a peak serum level of 43 mcg/mL after 5 minutes with a half-life of 20 to 30 minutes.
Oxacillin sodium, with normal doses, has insignificant concentrations in the cerebrospinal and ascitic fluids. It is found in therapeutic concentrations in the pleural, bile, and amniotic fluids.
Oxacillin Sodium is rapidly excreted as unchanged drug in the urine by glomerular filtration and active tubular secretion. The elimination half-life for oxacillin is about 0.5 hours. Nonrenal elimination includes hepatic inactivation and excretion in bile.
Oxacillin sodium binds to serum protein, mainly albumin. The degree of protein binding reported varies with the method of study and the investigator, but generally has been found to be 94.2 ± 2.1%.
Probenecid blocks the renal tubular secretion of penicillins. Therefore, the concurrent administration of probenecid prolongs the elimination of oxacillin and, consequently, increases the serum concentration.
Intramuscular injections give peak serum levels 30 minutes after injection. A 250 mg dose gives a level of 5.3 mcg/mL while a 500 mg dose peaks at 10.9 mcg/mL. Intravenous injection gives a peak about 5 minutes after the injection is completed. Slow IV dosing with 500 mg gives a 5 minute peak of 43 mcg/mL with a half-life of 20 to 30 minutes.
Penicillinase-resistant penicillins exert a bactericidal action against penicillin susceptible microorganisms during the state of active multiplication. All penicillins inhibit the biosynthesis of the bacterial cell wall.
Resistance to penicillins may be mediated by destruction of the beta-lactam ring by a beta-lactamase, altered affinity of penicillin for target, or decreased penetration of the antibiotic to reach the target site.
Resistance to oxacillin (or cefoxitin) implies resistance to all other beta-lactam agents, except newer agents with activity against methicillin-resistant Staphylococcus aureus.
When available, the clinical microbiology laboratory should provide of
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized test method1,2(broth and/or agar). The MIC values should be interpreted according to the criteria in Table 1.
Quantitative methods that require measurement of zone diameters can also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. The zone size should be determined using a standardized method.2,3.It has been determined that the most accurate method to test the susceptibility of microorganisms to penicillinase resistant penicillins, including oxacillin, by disk diffusion is achieved using disks impregnated with 30 mcg cefoxitin. Interpretation involves correlation of the diameter obtained with the cefoxitin disk test with the MIC for oxacillin2. Reports from the laboratory providing results of the standard single-disk susceptibility test with a 30 microgram cefoxitin disk should be interpreted according to the following criteria in Table 1.
Pathogen | Antimicrobial | Disk | Disk Diffusion Zone Diameter | Minimum Inhibitory | ||||
Agent | Content | (mm)a | Concentrations (mcg/mL) | |||||
S | I | R | S | I | R | |||
Staphylococcus aureus and S. lugdenensisC | Oxacillin | - | - | - | - | ≤ 2 (oxacillin) | - | ≥ 4 (oxacillin) |
30 mcg cefoxitinb | ≥ 22 | - | ≤ 21 | ≤ 4 (cefoxitin) | - | ≥ 8 (cefoxitin) | ||
Coagulase- negative Staphylococci except S.lugdenensis | Oxacillind | - | - | - | - | ≤ 0.25 | - | ≥ 0.5 |
30 mcg cefoxitinb | ≥25 | - | ≤24 | - | - | - | ||
S=susceptible, I=intermediate, R-resistant
- aIn most staphylococcal isolates, oxacillin resistance is mediated by mecA, encoding the penicillin binding protein 2a (PBP2a, also called PBP2’). Isolates that test positive for mecA or PBP2a should be reported as oxacillin resistant.”
- 2bCefoxitin is used as a surrogate for oxacillin; report oxacillin susceptible or resistant based on the cefoxitin result.
- 2cIf both cefoxitin and oxacillin are tested againstS. aureusorS. lugdenensis, and either result is resistant, the organism should be reported as oxacillin resistant.
- 2dOxacillin MIC interpretive criteria may overcall resistance for some coagulase-negative staphylococci (CoNS), because some non-S. epidermidisstrains for which the oxacillin MICs are 0.5 to 2 mcg/ml lackmecA. For serious infections with CoNS other thanS. epidermidis, testing formecAor for PBP 2a or with cefoxitin disk diffusion
A report of “Susceptible” indicates that the antimicrobial is likely to inhibit the growth of the pathogen if the antimicrobial compound reaches the concentrations at the infection site necessary to inhibit growth of the pathogen. A report of “Intermediate” indicates that the result should be considered equivocal, and if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated. This category also provides a buffer zone that prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of “Resistant” indicates that the antimicrobial is not likely to inhibit growth of the pathogen if the antimicrobial compound reaches the concentrations usually achievable at the infection site; other therapy should be selected.
Quality Control
Standardized susceptibility test procedures require the use of laboratory controls to monitor and ensure the accuracy and precision of the supplies and reagents used in the assay, and the techniques of the individuals performing the test.1,2,3Standard oxacillin powder should provide the following range of MIC values 1 noted in Table 2. For the diffusion technique using the 30 mcg cefoxitin disk, the criteria in Table 2 should be achieved.
Quality Control Organism | Minimum Inhibitory Concentration (mcg/mL) | Disk Diffusion Zone Diameters (mm) |
Enterococcus faecalis ATCC ® 29212 | 8-32 | - |
Staphylococcus aureus ATCC® 25923 | - | 18-24 |
Staphylococcus aureus ATCC ® 29213 | 0.12 - 0.5 | - |
Streptococcus pneumonia ATCC® 49619a | - | ≤12b |
ATCC=American Type Culture Collection
aDespite the lack of reliable disk diffusion interpretive criteria for
bDeterioration of oxacillin disk content is best assessed with QC organisms
When available, the clinical microbiology laboratory should provide of
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized test method1,2(broth and/or agar). The MIC values should be interpreted according to the criteria in Table 1.
Quantitative methods that require measurement of zone diameters can also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. The zone size should be determined using a standardized method.2,3.It has been determined that the most accurate method to test the susceptibility of microorganisms to penicillinase resistant penicillins, including oxacillin, by disk diffusion is achieved using disks impregnated with 30 mcg cefoxitin. Interpretation involves correlation of the diameter obtained with the cefoxitin disk test with the MIC for oxacillin2. Reports from the laboratory providing results of the standard single-disk susceptibility test with a 30 microgram cefoxitin disk should be interpreted according to the following criteria in Table 1.
Pathogen | Antimicrobial | Disk | Disk Diffusion Zone Diameter | Minimum Inhibitory | ||||
Agent | Content | (mm)a | Concentrations (mcg/mL) | |||||
S | I | R | S | I | R | |||
Staphylococcus aureus and S. lugdenensisC | Oxacillin | - | - | - | - | ≤ 2 (oxacillin) | - | ≥ 4 (oxacillin) |
30 mcg cefoxitinb | ≥ 22 | - | ≤ 21 | ≤ 4 (cefoxitin) | - | ≥ 8 (cefoxitin) | ||
Coagulase- negative Staphylococci except S.lugdenensis | Oxacillind | - | - | - | - | ≤ 0.25 | - | ≥ 0.5 |
30 mcg cefoxitinb | ≥25 | - | ≤24 | - | - | - | ||
S=susceptible, I=intermediate, R-resistant
- aIn most staphylococcal isolates, oxacillin resistance is mediated by mecA, encoding the penicillin binding protein 2a (PBP2a, also called PBP2’). Isolates that test positive for mecA or PBP2a should be reported as oxacillin resistant.”
- 2bCefoxitin is used as a surrogate for oxacillin; report oxacillin susceptible or resistant based on the cefoxitin result.
- 2cIf both cefoxitin and oxacillin are tested againstS. aureusorS. lugdenensis, and either result is resistant, the organism should be reported as oxacillin resistant.
- 2dOxacillin MIC interpretive criteria may overcall resistance for some coagulase-negative staphylococci (CoNS), because some non-S. epidermidisstrains for which the oxacillin MICs are 0.5 to 2 mcg/ml lackmecA. For serious infections with CoNS other thanS. epidermidis, testing formecAor for PBP 2a or with cefoxitin disk diffusion
A report of “Susceptible” indicates that the antimicrobial is likely to inhibit the growth of the pathogen if the antimicrobial compound reaches the concentrations at the infection site necessary to inhibit growth of the pathogen. A report of “Intermediate” indicates that the result should be considered equivocal, and if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated. This category also provides a buffer zone that prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of “Resistant” indicates that the antimicrobial is not likely to inhibit growth of the pathogen if the antimicrobial compound reaches the concentrations usually achievable at the infection site; other therapy should be selected.
Quality Control
Standardized susceptibility test procedures require the use of laboratory controls to monitor and ensure the accuracy and precision of the supplies and reagents used in the assay, and the techniques of the individuals performing the test.1,2,3Standard oxacillin powder should provide the following range of MIC values 1 noted in Table 2. For the diffusion technique using the 30 mcg cefoxitin disk, the criteria in Table 2 should be achieved.
Quality Control Organism | Minimum Inhibitory Concentration (mcg/mL) | Disk Diffusion Zone Diameters (mm) |
Enterococcus faecalis ATCC ® 29212 | 8-32 | - |
Staphylococcus aureus ATCC® 25923 | - | 18-24 |
Staphylococcus aureus ATCC ® 29213 | 0.12 - 0.5 | - |
Streptococcus pneumonia ATCC® 49619a | - | ≤12b |
ATCC=American Type Culture Collection
aDespite the lack of reliable disk diffusion interpretive criteria for
bDeterioration of oxacillin disk content is best assessed with QC organisms
Oxacillin may be used to initiate therapy in suspected cases of resistant staphylococcal infections prior to the availability of susceptibility test results. Oxacillin should not be used in infections caused by organisms susceptible to penicillin G. If the susceptibility tests indicate that the infection is due to an organism other than a resistant
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Oxacillin for Injection, USP and other antibacterial drugs, Oxacillin for Injection, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Bacteriologic studies to determine the causative organisms and their susceptibility to oxacillin should always be performed. Duration of therapy varies with the type of severity of infection as well as the overall condition of the patient; therefore, it should be determined by the clinical and bacteriological response of the patient. In severe staphylococcal infections, therapy with oxacillin should be continued for at least 14 days. Therapy should be continued for at least 48 hours after the patient has become afebrile, asymptomatic, and cultures are negative. Treatment of endocarditis and osteomyelitis may require a longer duration of therapy.
With intravenous administration, particularly in elderly patients, care should be taken because of the possibility of thrombophlebitis.
Drug | Adults | Infants and Children < 40 kg (88 lbs) | Other Recommendations |
Oxacillin | 250 to 500 mg IM or IV every 4 to 6 hours (mild to moderate infections) | 50 mg/kg/day IM or IV in equally divided doses every 6 hours (mild to moderate infections) | |
1 gram IM or IV every 4 to 6 hours (severe infections) | 100 mg/kg/day IM or IV in equally divided doses every 4 to 6 hours (severe infections) | Premature and Neonates 25 mg/kg/day IM or IV |
A history of a hypersensitivity (anaphylactic) reaction to any penicillin is a contraindication.
The reported incidence of allergic reactions to penicillin ranges from 0.7 to 10 percent (see
Serious and occasionally fatal hypersensitivity (anaphylactic shock with collapse) reactions have occurred in patients receiving penicillin. The incidence of anaphylactic shock in all penicillin-treated patients is between 0.015 and 0.04 percent. Anaphylactic shock resulting in death has occurred in approximately 0.002 percent of the patients treated.
When oxacillin therapy is indicated, it should be initiated only after a comprehensive patient drug and allergy history has been obtained. If an allergic reaction occurs, oxacillin should be discontinued and appropriate therapy instituted.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against
Two types of allergic reactions to penicillins are noted clinically, immediate and delayed.
Immediate reactions usually occur within 20 minutes of administration and range in severity from urticaria and pruritus to angioneurotic edema, laryngospasm, bronchospasm, hypotension, vascular collapse and death. Such immediate anaphylactic reactions are very rare (see
Serious and occasionally fatal hypersensitivity (anaphylactic shock with collapse) reactions have occurred in patients receiving penicillin. The incidence of anaphylactic shock in all penicillin-treated patients is between 0.015 and 0.04 percent. Anaphylactic shock resulting in death has occurred in approximately 0.002 percent of the patients treated.
When oxacillin therapy is indicated, it should be initiated only after a comprehensive patient drug and allergy history has been obtained. If an allergic reaction occurs, oxacillin should be discontinued and appropriate therapy instituted.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against
Delayed allergic reactions to penicillin therapy usually occur after 48 hours and sometimes as late as 2 to 4 weeks after initiation of therapy.
Manifestations of this type of reaction include serum sickness-like symptoms (
Tetracycline, a bacteriostatic antibiotic, may antagonize the bactericidal effect of penicillin and concurrent use of these drugs should be avoided.
Oxacillin blood levels may be increased and prolonged by concurrent administration of probenecid which blocks the renal tubular secretion of penicillins. Probenecid decreases the apparent volume of distribution and slows the rate of excretion by competitively inhibiting renal tubular secretion of penicillins.
Oxacillin-probenecid therapy should be limited to those infections where very high serum levels of oxacillin are necessary.
Oxacillin for Injection, USP is a semisynthetic penicillin antibiotic derived from the penicillin nucleus, 6-amino-penicillanic acid. It is resistant to inactivation by the enzyme penicillinase (beta-lactamase). It is the sodium salt in parenteral dosage form for intramuscular or intravenous use.
Each vial of oxacillin for injection, USP contains oxacillin sodium monohydrate equivalent to 1 gram or 2 grams of oxacillin. The sodium content is 57.4 mg (2.5 mEq) per gram oxacillin. The product is buffered with 20 mg dibasic sodium phosphate per gram oxacillin. Oxacillin for injection, USP is white to off white powder and gives a clear solution upon reconstitution.
OXACILLIN SODIUM
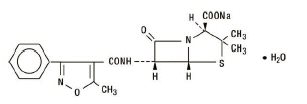
The chemical name of oxacillin sodium is 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 3,3-dimethyl-6-[[(5-methyl-3-phenyl-4-isoxazolyl)carbonyl]-amino]-7-oxo-, monosodium salt, monohydrate, [2S-(2α,5α,6ß)]-. It is resistant to inactivation by the enzyme penicillinase (beta-lactamase). The molecular formula of oxacillin sodium is C
19H
18N
3NaO
5S•H
2O. The molecular weight is 441.43.