Oxlumo
(Lumasiran)Dosage & Administration
| Body Weight | Loading Dose | Maintenance Dose |
|---|---|---|
| less than 10 kg | 6 mg/kg once monthly for 3 doses | 3 mg/kg once monthly, beginning 1 month after the last loading dose |
| 10 kg to less than 20 kg | 6 mg/kg once monthly for 3 doses | 6 mg/kg once every 3 months (quarterly), beginning 1 month after the last loading dose |
| 20 kg and above | 3 mg/kg once monthly for 3 doses | 3 mg/kg once every 3 months (quarterly), beginning 1 month after the last loading dose |
OXLUMO is intended for subcutaneous use and should be administered by a healthcare professional.
Visually inspect the drug product solution. Do not use if it contains particulate matter or if it is cloudy or discolored. OXLUMO is a sterile, preservative-free, clear, colorless-to-yellow solution. It is supplied in a single-dose vial, as a ready-to-use solution that does not require additional reconstitution or dilution prior to administration.
By using PrescriberAI, you agree to the AI Terms of Use.
Oxlumo Prescribing Information
OXLUMO is indicated for the treatment of primary hyperoxaluria type 1 (PH1) to lower urinary and plasma oxalate levels in pediatric and adult patients
Lumasiran reduces levels of glycolate oxidase (GO) enzyme by targeting the hydroxyacid oxidase 1 (
ILLUMINATE-A was a randomized, double-blind trial comparing lumasiran and placebo in 39 patients 6 years of age and older with PH1 and an eGFR ≥30 mL/min/1.73 m2(ILLUMINATE-A; NCT03681184). Patients received 3 loading doses of 3 mg/kg OXLUMO (N=26) or placebo (N=13) administered once monthly, followed by quarterly maintenance doses of 3 mg/kg OXLUMO or placebo
The median age of patients at first dose was 15 years (range 6 to 61 years), 67% were male, and 77% were White. At baseline, the median 24-hour urinary oxalate excretion corrected for body surface area (BSA) was 1.7 mmol/24 h/1.73 m2, the median plasma oxalate level was 13.1 µmol/L, 33% of patients had eGFR ≥90 mL/min/1.73 m2, 49% had eGFR of 60 to <90 mL/min/1.73 m2, and 18% had eGFR 30 to <60 mL/min/1.73 m2, 56% were on pyridoxine, and 85% reported a history of symptomatic kidney stone events.
The primary endpoint was the percent reduction from baseline in 24-hour urinary oxalate excretion corrected for BSA averaged over Months 3 through 6. The LS mean percent change from baseline in 24-hour urinary oxalate in the OXLUMO group was -65% (95% CI: -71, -59) compared with -12% (95% CI: -20, -4) in the placebo group, resulting in a between-group LS mean difference of 53% (95% CI: 45, 62; p<0.0001) [Figure 1].
| Abbreviation: CI ꞊ Confidence Interval. Results are plotted as mean (95% CI) of percent change from baseline. |
Figure 1. ILLUMINATE-A: Percent Change from Baseline in 24-hour Urinary Oxalate by Month |
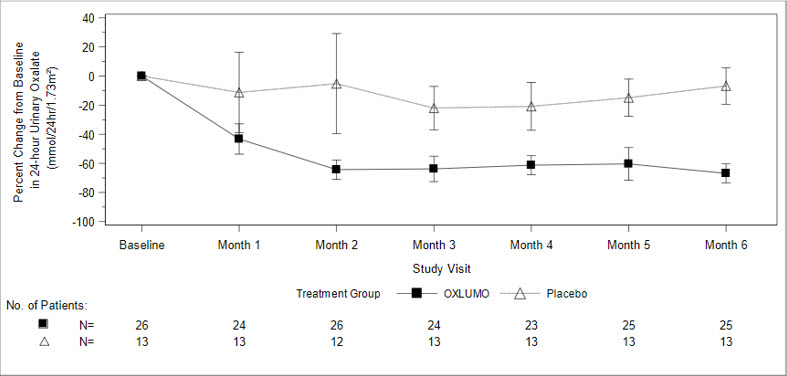 |
By Month 6, 52% (95% CI: 31, 72) of patients treated with OXLUMO achieved a normal 24-hour urinary oxalate corrected for BSA (≤0.514 mmol/24 hr/1.73 m2) compared to 0% (95% CI: 0, 25) placebo-treated patients (p=0.001). Reduced urinary oxalate levels were maintained through Month 24 in patients treated with OXLUMO.

ILLUMINATE-B was a single-arm study in 18 patients <6 years of age with PH1 and an eGFR >45 mL/min/1.73 m2for patients ≥12 months of age or a normal serum creatinine for patients <12 months of age (ILLUMINATE-B; NCT03905694). Dosing was based on body weight
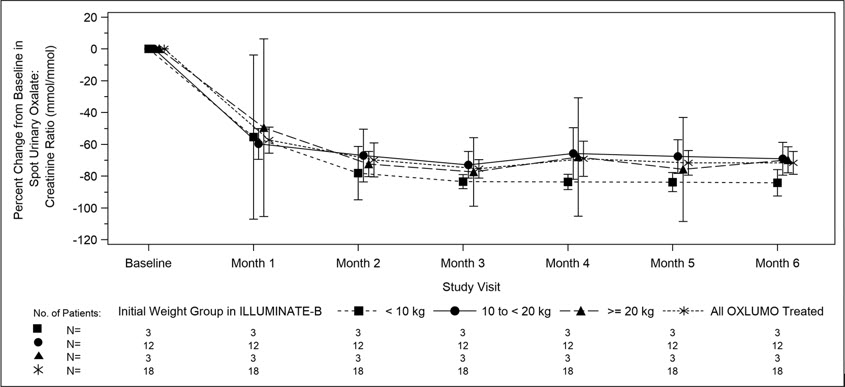
The median age of patients at first dose was 51 months (range 4 to 74 months), 56% were female, and 88% were White. Three patients were less than 10 kg, 12 were 10 kg to <20 kg, and 3 were ≥20 kg. The median spot urinary oxalate: creatinine ratio at baseline was 0.47 mmol/mmol.
The primary endpoint was the percent reduction from baseline in spot urinary oxalate: creatinine ratio averaged over Months 3 through 6. Patients treated with OXLUMO achieved a reduction in spot urinary oxalate: creatinine ratio from baseline of 72% (95% CI: 66, 78) (Figure 2). The reduction in urinary oxalate excretion was maintained with continued OXLUMO treatment through Month 12.
| Abbreviation: CI ꞊ Confidence Interval. Results are plotted as mean (95% CI) of percent change from baseline. |
Figure 2. ILLUMINATE-B: Percent Change from Baseline in Spot Urinary Oxalate: Creatinine Ratio by Month |
|
A total of 21 patients were enrolled and treated with OXLUMO in a multi-center, single-arm study in patients with PH1 and an eGFR ≤45 mL/min/1.73 m2in patients 12 months of age and older or an elevated serum creatinine for age in patients less than 12 months of age, including patients on hemodialysis. ILLUMINATE-C included 2 cohorts. Cohort A included 6 patients who did not require dialysis at the time of study enrollment. Cohort B included 15 patients who were on a stable regimen of hemodialysis; the hemodialysis regimen was to remain stable in these patients for the first 6 months of the study. Patients received the recommended dosing regimen of OXLUMO based on body weight
The median age of patients at first dose was 9 years (range 0 to 59 years), 57% were male, and 76% were White. For Cohort A, the median plasma oxalate level was 58 µmol/L. For Cohort B, the median pre-dialysis plasma oxalate level was 104 µmol/L.
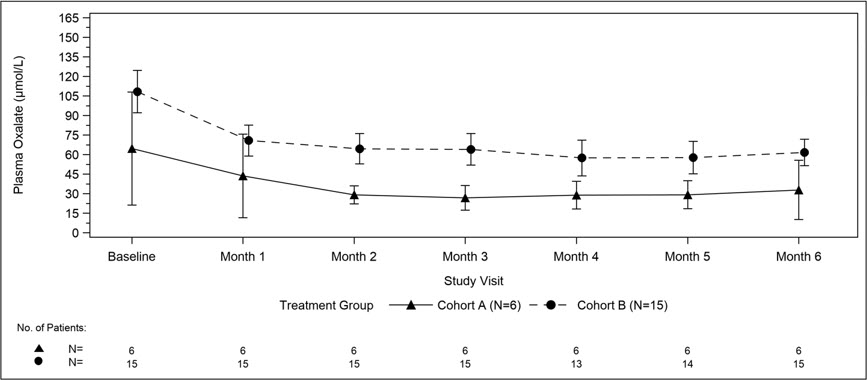
The primary endpoint was the percent change in plasma oxalate from baseline to Month 6 (average from Month 3 to Month 6) for Cohort A (N=6) and the percent change in pre-dialysis plasma oxalate from baseline to Month 6 (average from Month 3 to Month 6) for Cohort B (N=15). The percent change from baseline to Month 6 in plasma oxalate levels in Cohort A was an LS mean difference of -33% (95% CI: -82, 15) and in Cohort B was -42% (95% CI: -51, -34).
Mean plasma oxalate decreased from 65 µmol/L (95% CI: 21, 108) at baseline to 33 µmol/L (95% CI: 10, 56) at Month 6 in Cohort A, and from 108 µmol/L (95% CI: 92, 125) at baseline to 62 µmol/L (95% CI: 51, 72) at Month 6 in Cohort B. The time course for changes in plasma oxalate is shown in Figure 3.
| Abbreviation: CI ꞊ Confidence Interval. Results are plotted as mean (95% CI) of actual values. For Cohort A, the baseline is defined as the mean of all plasma oxalate samples collected prior to the first dose of lumasiran; for Cohort B, the baseline is defined as the last four pre-dialysis plasma oxalate samples collected prior to the first dose of lumasiran. In Cohort B, only pre-dialysis samples are utilized. |
Figure 3. ILLUMINATE-C: Plasma Oxalate Levels (µmol/L) during the Primary Analysis Period by Month |
 |

- The recommended dose of OXLUMO by subcutaneous injection is based on body weight. ()
2.1 Recommended DosageThe recommended dosing regimen of OXLUMO consists of loading doses (monthly for 3 doses) followed by maintenance doses (beginning 1 month after the last loading dose) administered subcutaneously as shown in Table 1.
Dosing is based on actual body weight.
Table 1. OXLUMO Weight-Based Dosing Regimen Body Weight Loading Dose Maintenance Dose Less than 10 kg 6 mg/kg once monthly for 3 doses 3 mg/kg once monthly, beginning 1 month after the last loading dose 10 kg to less than 20 kg 6 mg/kg once monthly for 3 doses 6 mg/kg once every 3 months (quarterly), beginning 1 month after the last loading dose 20 kg and above 3 mg/kg once monthly for 3 doses 3 mg/kg once every 3 months (quarterly), beginning 1 month after the last loading dose For Patients on HemodialysisAdminister OXLUMO after hemodialysis if administered on dialysis days.
Missed DoseIf a dose is delayed or missed, administer OXLUMO as soon as possible. Resume prescribed monthly or quarterly dosing, from the most recently administered dose.
| Body Weight | Loading Dose | Maintenance Dose |
|---|---|---|
| less than 10 kg | 6 mg/kg once monthly for 3 doses | 3 mg/kg once monthly, beginning 1 month after the last loading dose |
| 10 kg to less than 20 kg | 6 mg/kg once monthly for 3 doses | 6 mg/kg once every 3 months (quarterly), beginning 1 month after the last loading dose |
| 20 kg and above | 3 mg/kg once monthly for 3 doses | 3 mg/kg once every 3 months (quarterly), beginning 1 month after the last loading dose |
- See Full Prescribing Information for important preparation and administration instructions. ()
2.2 Administration InstructionsOXLUMO is intended for subcutaneous use and should be administered by a healthcare professional.
Visually inspect the drug product solution. Do not use if it contains particulate matter or if it is cloudy or discolored. OXLUMO is a sterile, preservative-free, clear, colorless-to-yellow solution. It is supplied in a single-dose vial, as a ready-to-use solution that does not require additional reconstitution or dilution prior to administration.
- Use aseptic technique.
- Divide injection volumes greater than 1.5 mL equally into multiple syringes.
- For volumes less than 0.3 mL, a sterile 0.3-mL syringe is recommended. If using a 0.3 mL (30 unit) insulin syringe, 1-unit markings indicate 0.01 mL.
- Administer subcutaneous injection into the abdomen, thigh, or the side or back of the upper arms. Rotate injection sites. Do not inject into scar tissue or areas that are reddened, inflamed, or swollen.
- If injecting into the abdomen, avoid the area around the navel.
- If more than one injection is needed for a single dose of OXLUMO, the injection sites should be at least 2 cm apart.
- Discard unused portion of the drug.
Injection: 94.5 mg/0.5 mL clear, colorless-to-yellow solution in a single-dose vial.
There are no available data with the use of OXLUMO in pregnant women to evaluate a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes.
No adverse effects on pregnancy or embryo-fetal development related to OXLUMO were observed in rats at 45 times and in rabbits at 90 times the maximum recommended human dose in women (see
In an embryo-fetal development study in pregnant rats, lumasiran was administered subcutaneously at doses of 3, 10, and 30 mg/kg/day during organogenesis (gestational days 6-17). Administration of lumasiran resulted in no effects on embryo-fetal survival or fetal body weights and no lumasiran-related fetal malformations were observed. The 30 mg/kg/day dose in rats is 45 times the maximum recommended human dose (MRHD) for women of 3 mg/kg/month normalized to 0.1 mg/kg/day, based on body surface area. In an embryo-fetal development study in female rabbits, lumasiran was administered subcutaneously at doses of 3, 10, and 30 mg/kg/day during organogenesis (gestational days 7-19). There were decreases in maternal food consumption and decreases in maternal body weight gains at doses ≥3 mg/kg/day. There were no lumasiran-related fetal findings identified at doses up to 30 mg/kg/day (90 times the normalized MRHD based on body surface area).
In a postnatal development study, lumasiran administered subcutaneously to pregnant female rats on gestational days 7, 13, 19 and on lactation days 6, 12, and 18 through weaning at doses up to 50 mg/kg did not produce maternal toxicity or developmental effects in the offspring.
The estimated background risk of major birth defects and miscarriage in the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
None.
The most common adverse reaction (reported in ≥20% of patients) is injection site reactions. (
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of OXLUMO has been evaluated in a placebo-controlled trial and two single-arm clinical trials. Across these trials, 98 patients with PH1 have been treated with OXLUMO, including 71 pediatric patients and 15 patients on hemodialysis. Overall, 92 patients were treated for at least 6 months, 78 patients for at least 12 months, and 29 patients for at least 24 months.
In the randomized, placebo-controlled, double-blind study ILLUMINATE-A in pediatric and adult patients with PH1 aged 6 to 61 years, 26 patients received OXLUMO, and 13 patients received placebo. Of these, 25 patients received ≥5 months of treatment.
In two single-arm studies in patients with PH1, ILLUMINATE-B (patients <6 years of age) and ILLUMINATE-C (pediatric and adult patients with moderately or severely reduced GFR [eGFR ≤45 mL/min/1.73 m2or pediatric patients <12 months of age with serum creatinine above the upper limit of normal for age] and patients with kidney failure on hemodialysis), the OXLUMO safety profile was similar to that seen in ILLUMINATE-A
In placebo-controlled and open-label clinical studies the most common adverse reaction reported was injection site reaction. Injection site reactions included erythema, swelling, pain, hematoma, pruritus, and discoloration. These symptoms were generally mild and resolved within one day of the injection and did not lead to discontinuation of treatment.
| Adverse Reaction | OXLUMO N=26 N (%) | Placebo N=13 N (%) |
|---|---|---|
| Injection site reaction | 10 (38) | 0 (0) |
| Abdominal painGrouped term includes abdominal pain, abdominal pain upper, abdominal pain lower, and abdominal discomfort. | 4 (15) | 1 (8) |