Get your patient on Paragard Iud (Copper)
Paragard IUD patient education
Patient toolkit
Dosage & administration
2 DOSAGE AND ADMINISTRATION
- Insert a single Paragard at the fundus of the uterine cavity. Remove Paragard no later than 10 years from the date of insertion. (2.1 )
- Insert and remove Paragard only if you are a healthcare provider trained on these procedures. (2.1 )
- See the Full Prescribing Information for recommended timing of insertion preparation instructions, insertion procedures, postplacement management, and instructions on removing Paragard (2.2 , 2.3 , 2.4 , 2.5 , 2.6 )
- Following the insertion, examine the patient after her first menses to confirm Paragard is still in place. (2.5 )
Important Dosage and Administration Instructions
- Paragard should only be inserted by a healthcare provider trained in Paragard’s insertion procedures, because insertion for Paragard is different from that used for other intrauterine systems. Healthcare providers should become thoroughly familiar with the product, product educational materials, product insertion instructions, and prescribing information before attempting insertion of Paragard.
- Insert one Paragard at the fundus of the uterine cavity [see Dosage and Administration (2.4 )].
- Remove Paragard on or before 10 years from the date of insertion [see Dosage and Administration (2.6 )].
- May replace Paragard at the time of removal with a new Paragard if continued contraceptive protection is desired.
- Before considering use of Paragard, make sure that the female is an appropriate candidate for Paragard. Exclude pregnancy (consider the possibility of ovulation and conception) prior to use [see Contraindicati ons (4 ) and Warnings and Precautions (5.2 )].
Timing of Insertion
Refer to Table 1 for recommended timing of Paragard insertion.
| Clinical Situation | Recommended Timing of Paragard Insertion |
| 1. Start Paragard in females not currently using contraception | At any time during the menstrual cycle. |
| 2. Switch to Paragard from an oral, transdermal, or vaginal form of hormonal contraception or an injectable progestin contraceptive | At any time during the menstrual cycle; discontinue the previous method. |
| 3. Switch to Paragard from a contraceptive implant or other intrauterine system | Same day the implant or IUS is removed (insert at any time during the menstrual cycle). |
| 4. Insert Paragard after abortion or miscarriage | Immediately after abortion, although immediate placement has a slightly higher risk of expulsion than |
| placement at other times. Insertion after second trimester abortion is associated with a higher risk of expulsion than insertion after a first trimester abortion. | |
| 5. Insert Paragard after Childbirth | May insert immediately postpartum. |
| Insertion before uterine involution is complete, which may not occur until the second postpartum month, has been associated with increased risk of expulsion [see Warnings and Precautions (5.6 , 5.7 )]. | |
| There appears to be an increased risk of perforation in lactating women [see Warnings and Precautions (5.6 )]. | |
Preparation Instruction
Before insertion:
- Use strict aseptic techniques throughout preparation [see Warnings and Precautions (5.4 )].
- Prepare placement tools (e.g., speculum, cotton swab, tenaculum, uterine sound, scissors, and forceps).
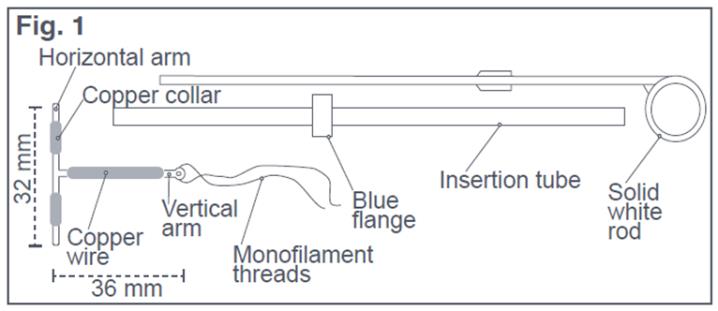
- Place the package containing Paragard (face-up), sterile card, and solid white rod on a sterile field (see Figure 1) and open package from the bottom end where arrow says “open”.
Figure 1: Paragard Intrauterine System (IUS) with Insertion Tube and Solid White Rod
- Consider the use of an analgesic
- Establish the size and position of the uterus by performing a bi-manual examination.
- Insert a speculum and, using a cotton swab, cleanse the cervix and vagina with an antiseptic solution.
- Apply a tenaculum to the cervix and use gentle traction to align the cervical canal with the uterine cavity.
- Gently insert a sterile uterine sound to measure the depth of the uterine cavity. The uterus should sound to a depth of 6 to 9 cm except when inserting Paragard immediately postabortion or immediately postpartum.
- Insertion of Paragard may be associated with pain and/or bleeding or vasovagal reactions (e.g. syncope, bradycardia, or seizure) especially in patients with a predisposition to these symptoms. Insertion into a uterine cavity measuring less than 6 cm may increase the incidence of expulsion, bleeding, pain, and perforation.
- If cervical stenosis is encountered, avoid undue force. Dilators and analgesia/local anesthesia may be helpful in this situation.
- Insertion of Paragard may be associated with pain and/or bleeding or vasovagal reactions (e.g. syncope, bradycardia, or seizure) especially in patients with a predisposition to these symptoms. Insertion into a uterine cavity measuring less than 6 cm may increase the incidence of expulsion, bleeding, pain, and perforation.
Insertion Procedure
- Use strict aseptic techniques throughout the insertion procedure [see Warnings and Precautions (5.4 )]. Using sterile gloves, bend the T-Arms of Paragard by folding the two horizontal arms down against the stem.
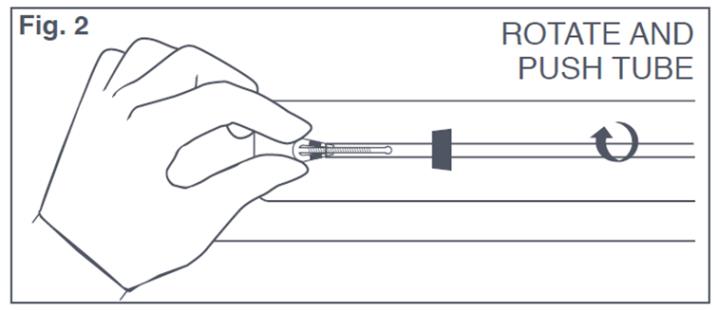
- Slightly withdraw insertion tube, push arms down along the stem, slide insertion tube over the tips of the T-Arms (see Figure 2). Only the tips of the T-Arms should be in the insertion tube. Do not advance beyond the copper collars. Insert solid white rod into bottom of insertion tube until it touches the bottom of the IUS. Do not leave the horizontal arms of Paragard bent for more than 5 minutes, as the arms may not open properly.
Figure 2: Inserting Tips of T-Arms of Paraguard into Insertion Tube
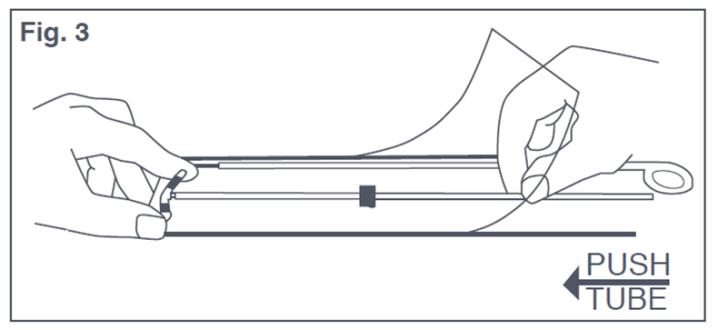
- Although using sterile gloves is recommended, in situations where sterile gloves are not available, you can perform this step while Paragard is in the sterile package. Place the package face up on a clean surface. Open from the bottom end where arrow says “open”. Pull the solid white rod from the package and put it back in the package laying it carefully alongside the insertion tube, making sure the distal end of the rod remains sterile. Place thumb and index finger on the outside of the package, on top of the ends of the horizontal arms. Use other hand to push insertion tube against arms of Paragard (shown by arrow in Figure 3). This will start bending the T-Arms downward. Note that the arms of Paragard should be folded downward to ensure proper insertion.
Figure 3: Bending T-Arms of Paraguard While in Sterile Packaging
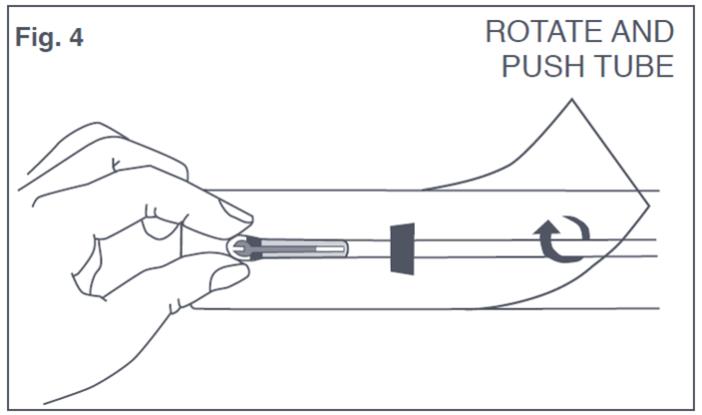
- Bring the thumb and index finger closer together on the outside of the package to continue bending the arms until they are alongside the stem. Use the other hand to withdraw the insertion tube slightly so that the insertion tube can be pushed and rotated over the tips of the T-Arms (see Figure 4). Only the tips of the T-Arms should be in the insertion tube. Do not advance beyond the copper collars. Insert solid white rod into bottom of the insertion tube until it touches the bottom of the IUS. Do not leave the horizontal arms of Paragard bent for more than 5 minutes, as the arms may not open properly.
Figure 4: Inserting Tips of T-Arms of Paraguard into Insertion Tube While in Sterile Packaging
- Once the above steps are completed and Paragard is in the insertion tube, grasp the insertion tube at the open end of the package; adjust the blue flange so that the distance from the top of the Paragard insertion tube is the same as the uterine depth measured with the uterine sound or use the sterile card to adjust the blue flange according to the premeasured uterine depth.
- Rotate the blue flange so that the horizontal arms of Paragard and the long axis of the blue flange lie in the same horizontal plane to ensure the arms open up in the proper direction.
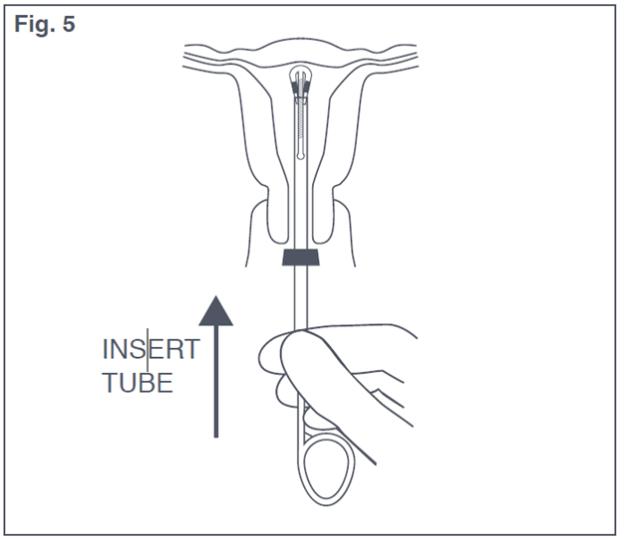
- To orient the uterus in an axial position, apply gentle traction to the tenaculum. Then pass the loaded insertion tube through the cervical canal until Paragard just touches the fundus of the uterus. The blue flange should be at the cervix in the horizontal plane (see Figure 5).
Figure 5: Insertion Tube with Paraguard in Uterus
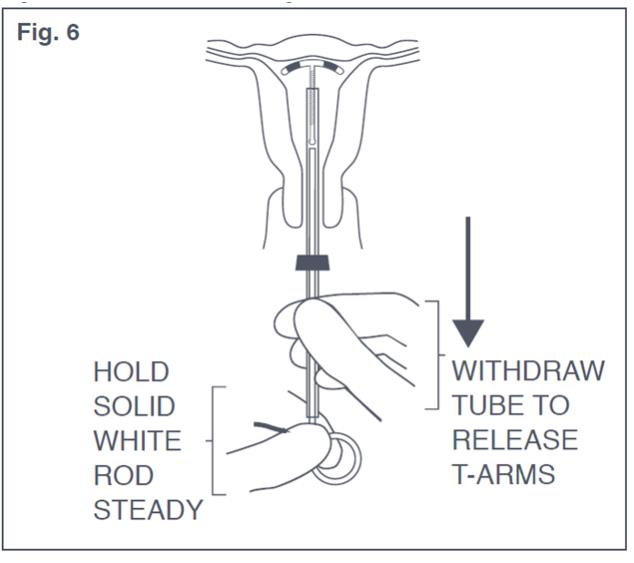
- Release the arms of Paragard by holding the solid white rod steady and withdrawing the insertion tube no more than one centimeter. This releases the arms of Paragard high in the uterine fundus (see Figure 6).
Figure 6: Release of T-Arms of Paraguard in Uterus
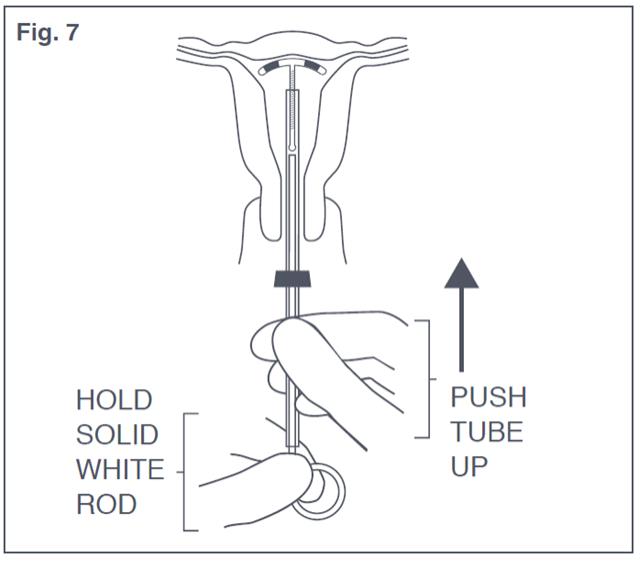
- Gently and carefully move the insertion tube upward toward the fundus of the uterus, until slight resistance is felt. This will ensure placement of Paragard at the highest possible position within the uterus (see Figure 7). Do not use the white rod as a plunger to push or insert Paragard.
Figure 7: Placement of Paraguard in Fundus of Uterus
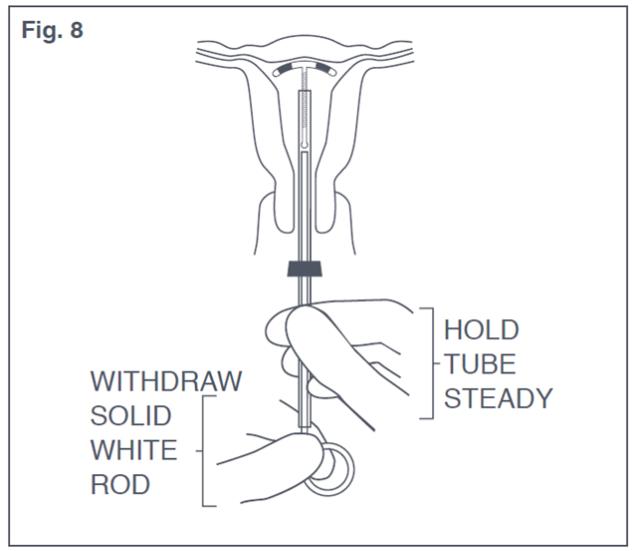
- Hold the insertion tube steady and withdraw the solid white rod (see Figure 8). Do not remove the solid white rod and the insertion tube at the same time to prevent accidental pulling of the threads.
Figure 8: Withdraw Solid White Rod from Uterus
- Gently and slowly withdraw the insertion tube from the cervical canal.
- Only the threads should be visible protruding from the cervix (see Figure 9). Trim the threads so that 3 to 4 cm protrude into the vagina. Measure the length of protrusion of the threads.
- Recommend recording length of threads, date of placement and Paragard lot number.
Figure 9: Appropriate Paraguard Placement in Uterus
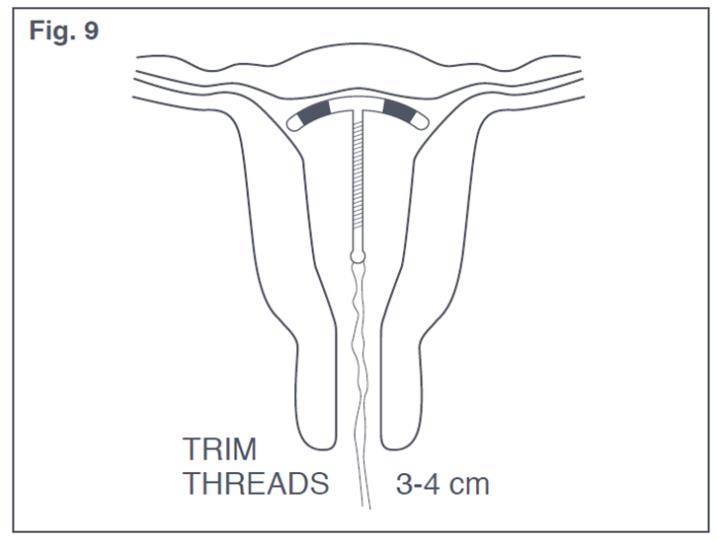
If you suspect that Paragard is not in the correct position, check placement (with ultrasound, if necessary). If Paragard is not positioned completely within the uterus, remove it and replace it with a new Paragard. Do not reinsert an expelled or partially expelled Paragard.
Postplacement Management of Paraguard
Following placement:
- Examine the female after her first menses to confirm that Paragard is still in place. You should be able to visualize or feel only the threads. The length of the visible threads may change with time. However, no action is needed unless you suspect partial expulsion, perforation, pregnancy, or breakage.
- If you cannot find the threads in the vagina, check that Paragard is still in the uterus. The threads can retract into the uterus or break, or Paragard can break, perforate the uterus, or be expelled. Gentle probing of the cavity, x-ray, or sonography may be required to locate Paragard
- Remove Paragard if it has been partially expelled or perforated the uterus [ see Warnings and Precautions (5.6 , 5.7 )].
Do not reinsert a used Paragard.
2.6 Removal of Paraguard
Timing of Removal
- Paragard can be removed at any time prior to 10 years after insertion.
- Remove Paragard no later than 10 years after insertion. A new Paragard can be inserted at the time of removal if continued contraceptive protection is desired.
Removal Instructions
- Use a speculum and visualize the cervix.
- Remove Paragard with forceps, pulling gently on the exposed threads. The arms of Paragard will fold upwards as it is withdrawn from the uterus.
- Breakage or embedment of Paragard in the myometrium can make removal difficult [see Warnings and Precautions, (5.5 )]. Analgesia, paracervical anesthesia, cervical dilation, alligator forceps or other grasping instrument, or hysteroscopy may assist in removing an embedded Paragard.
- Make sure Paragard is intact upon removal.
- Removal may be associated with some pain and/or bleeding or vasovagal reactions (e.g. syncope, bradycardia, seizures) especially in patients with a predisposition to these conditions.

By using PrescriberAI, you agree to the AI Terms of Use.
Paragard IUD prescribing information
1 INDICATIONS AND USAGE
Paragard is indicated for prevention of pregnancy in females of reproductive potential for up to 10 years.
2 DOSAGE AND ADMINISTRATION
- Insert a single Paragard at the fundus of the uterine cavity. Remove Paragard no later than 10 years from the date of insertion. (2.1 )
- Insert and remove Paragard only if you are a healthcare provider trained on these procedures. (2.1 )
- See the Full Prescribing Information for recommended timing of insertion preparation instructions, insertion procedures, postplacement management, and instructions on removing Paragard (2.2 , 2.3 , 2.4 , 2.5 , 2.6 )
- Following the insertion, examine the patient after her first menses to confirm Paragard is still in place. (2.5 )
Important Dosage and Administration Instructions
- Paragard should only be inserted by a healthcare provider trained in Paragard’s insertion procedures, because insertion for Paragard is different from that used for other intrauterine systems. Healthcare providers should become thoroughly familiar with the product, product educational materials, product insertion instructions, and prescribing information before attempting insertion of Paragard.
- Insert one Paragard at the fundus of the uterine cavity [see Dosage and Administration (2.4 )].
- Remove Paragard on or before 10 years from the date of insertion [see Dosage and Administration (2.6 )].
- May replace Paragard at the time of removal with a new Paragard if continued contraceptive protection is desired.
- Before considering use of Paragard, make sure that the female is an appropriate candidate for Paragard. Exclude pregnancy (consider the possibility of ovulation and conception) prior to use [see Contraindicati ons (4 ) and Warnings and Precautions (5.2 )].
Timing of Insertion
Refer to Table 1 for recommended timing of Paragard insertion.
| Clinical Situation | Recommended Timing of Paragard Insertion |
| 1. Start Paragard in females not currently using contraception | At any time during the menstrual cycle. |
| 2. Switch to Paragard from an oral, transdermal, or vaginal form of hormonal contraception or an injectable progestin contraceptive | At any time during the menstrual cycle; discontinue the previous method. |
| 3. Switch to Paragard from a contraceptive implant or other intrauterine system | Same day the implant or IUS is removed (insert at any time during the menstrual cycle). |
| 4. Insert Paragard after abortion or miscarriage | Immediately after abortion, although immediate placement has a slightly higher risk of expulsion than |
| placement at other times. Insertion after second trimester abortion is associated with a higher risk of expulsion than insertion after a first trimester abortion. | |
| 5. Insert Paragard after Childbirth | May insert immediately postpartum. |
| Insertion before uterine involution is complete, which may not occur until the second postpartum month, has been associated with increased risk of expulsion [see Warnings and Precautions (5.6 , 5.7 )]. | |
| There appears to be an increased risk of perforation in lactating women [see Warnings and Precautions (5.6 )]. | |
Preparation Instruction
Before insertion:
- Use strict aseptic techniques throughout preparation [see Warnings and Precautions (5.4 )].
- Prepare placement tools (e.g., speculum, cotton swab, tenaculum, uterine sound, scissors, and forceps).
- Place the package containing Paragard (face-up), sterile card, and solid white rod on a sterile field (see Figure 1) and open package from the bottom end where arrow says “open”.
Figure 1: Paragard Intrauterine System (IUS) with Insertion Tube and Solid White Rod

- Consider the use of an analgesic
- Establish the size and position of the uterus by performing a bi-manual examination.
- Insert a speculum and, using a cotton swab, cleanse the cervix and vagina with an antiseptic solution.
- Apply a tenaculum to the cervix and use gentle traction to align the cervical canal with the uterine cavity.
- Gently insert a sterile uterine sound to measure the depth of the uterine cavity. The uterus should sound to a depth of 6 to 9 cm except when inserting Paragard immediately postabortion or immediately postpartum.
- Insertion of Paragard may be associated with pain and/or bleeding or vasovagal reactions (e.g. syncope, bradycardia, or seizure) especially in patients with a predisposition to these symptoms. Insertion into a uterine cavity measuring less than 6 cm may increase the incidence of expulsion, bleeding, pain, and perforation.
- If cervical stenosis is encountered, avoid undue force. Dilators and analgesia/local anesthesia may be helpful in this situation.
Insertion Procedure
- Use strict aseptic techniques throughout the insertion procedure [see Warnings and Precautions (5.4 )]. Using sterile gloves, bend the T-Arms of Paragard by folding the two horizontal arms down against the stem.
- Slightly withdraw insertion tube, push arms down along the stem, slide insertion tube over the tips of the T-Arms (see Figure 2). Only the tips of the T-Arms should be in the insertion tube. Do not advance beyond the copper collars. Insert solid white rod into bottom of insertion tube until it touches the bottom of the IUS. Do not leave the horizontal arms of Paragard bent for more than 5 minutes, as the arms may not open properly.
Figure 2: Inserting Tips of T-Arms of Paraguard into Insertion Tube

- Although using sterile gloves is recommended, in situations where sterile gloves are not available, you can perform this step while Paragard is in the sterile package. Place the package face up on a clean surface. Open from the bottom end where arrow says “open”. Pull the solid white rod from the package and put it back in the package laying it carefully alongside the insertion tube, making sure the distal end of the rod remains sterile. Place thumb and index finger on the outside of the package, on top of the ends of the horizontal arms. Use other hand to push insertion tube against arms of Paragard (shown by arrow in Figure 3). This will start bending the T-Arms downward. Note that the arms of Paragard should be folded downward to ensure proper insertion.
Figure 3: Bending T-Arms of Paraguard While in Sterile Packaging

- Bring the thumb and index finger closer together on the outside of the package to continue bending the arms until they are alongside the stem. Use the other hand to withdraw the insertion tube slightly so that the insertion tube can be pushed and rotated over the tips of the T-Arms (see Figure 4). Only the tips of the T-Arms should be in the insertion tube. Do not advance beyond the copper collars. Insert solid white rod into bottom of the insertion tube until it touches the bottom of the IUS. Do not leave the horizontal arms of Paragard bent for more than 5 minutes, as the arms may not open properly.
Figure 4: Inserting Tips of T-Arms of Paraguard into Insertion Tube While in Sterile Packaging

- Once the above steps are completed and Paragard is in the insertion tube, grasp the insertion tube at the open end of the package; adjust the blue flange so that the distance from the top of the Paragard insertion tube is the same as the uterine depth measured with the uterine sound or use the sterile card to adjust the blue flange according to the premeasured uterine depth.
- Rotate the blue flange so that the horizontal arms of Paragard and the long axis of the blue flange lie in the same horizontal plane to ensure the arms open up in the proper direction.
- To orient the uterus in an axial position, apply gentle traction to the tenaculum. Then pass the loaded insertion tube through the cervical canal until Paragard just touches the fundus of the uterus. The blue flange should be at the cervix in the horizontal plane (see Figure 5).
Figure 5: Insertion Tube with Paraguard in Uterus

- Release the arms of Paragard by holding the solid white rod steady and withdrawing the insertion tube no more than one centimeter. This releases the arms of Paragard high in the uterine fundus (see Figure 6).
Figure 6: Release of T-Arms of Paraguard in Uterus

- Gently and carefully move the insertion tube upward toward the fundus of the uterus, until slight resistance is felt. This will ensure placement of Paragard at the highest possible position within the uterus (see Figure 7). Do not use the white rod as a plunger to push or insert Paragard.
Figure 7: Placement of Paraguard in Fundus of Uterus

- Hold the insertion tube steady and withdraw the solid white rod (see Figure 8). Do not remove the solid white rod and the insertion tube at the same time to prevent accidental pulling of the threads.
Figure 8: Withdraw Solid White Rod from Uterus

- Gently and slowly withdraw the insertion tube from the cervical canal.
- Only the threads should be visible protruding from the cervix (see Figure 9). Trim the threads so that 3 to 4 cm protrude into the vagina. Measure the length of protrusion of the threads.
- Recommend recording length of threads, date of placement and Paragard lot number.
Figure 9: Appropriate Paraguard Placement in Uterus

If you suspect that Paragard is not in the correct position, check placement (with ultrasound, if necessary). If Paragard is not positioned completely within the uterus, remove it and replace it with a new Paragard. Do not reinsert an expelled or partially expelled Paragard.
Postplacement Management of Paraguard
Following placement:
- Examine the female after her first menses to confirm that Paragard is still in place. You should be able to visualize or feel only the threads. The length of the visible threads may change with time. However, no action is needed unless you suspect partial expulsion, perforation, pregnancy, or breakage.
- If you cannot find the threads in the vagina, check that Paragard is still in the uterus. The threads can retract into the uterus or break, or Paragard can break, perforate the uterus, or be expelled. Gentle probing of the cavity, x-ray, or sonography may be required to locate Paragard
- Remove Paragard if it has been partially expelled or perforated the uterus [ see Warnings and Precautions (5.6 , 5.7 )].
Do not reinsert a used Paragard.
2.6 Removal of Paraguard
Timing of Removal
- Paragard can be removed at any time prior to 10 years after insertion.
- Remove Paragard no later than 10 years after insertion. A new Paragard can be inserted at the time of removal if continued contraceptive protection is desired.
Removal Instructions
- Use a speculum and visualize the cervix.
- Remove Paragard with forceps, pulling gently on the exposed threads. The arms of Paragard will fold upwards as it is withdrawn from the uterus.
- Breakage or embedment of Paragard in the myometrium can make removal difficult [see Warnings and Precautions, (5.5 )]. Analgesia, paracervical anesthesia, cervical dilation, alligator forceps or other grasping instrument, or hysteroscopy may assist in removing an embedded Paragard.
- Make sure Paragard is intact upon removal.
- Removal may be associated with some pain and/or bleeding or vasovagal reactions (e.g. syncope, bradycardia, seizures) especially in patients with a predisposition to these conditions.
3 DOSAGE FORMS AND STRENGTHS
Paragard is a T-frame copper-containing intrauterine system (IUS) consisting of a polyethylene frame with barium sulfate measuring 32 mm horizontally and 36 mm vertically, with approximately 176 mg of copper wire wrapped around the vertical arm and an approximately 68.7 mg copper wire collar placed on each side of the horizontal arm with a total exposed copper surface area is 380 ± 23 mm², packaged with an insertion tube with blue flange and solid white rod. A monofilament polyethylene thread is tied through the tip of the vertical arm resulting in two white threads, each at least 10.5 cm in length. Figure 1 displays the contents of the package [see Dosage and Administration (2.3 )].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Use of Paragard is contraindicated for use in pregnant females because there is no need for pregnancy prevention in a female who is already pregnant and Paragard may cause adverse pregnancy outcomes. If a female becomes pregnant with Paragard in place, there is an increased risk of miscarriage, sepsis, premature labor, and premature delivery [see Contraindications (4 ) and Warnings and Precautions (5.1 , 5.2 )]. Advise the female of the potential risks if pregnancy occurs with Paragard in place.
Published studies on pregnancy outcomes exposed to copper IUSs report up to 27% miscarriage when the IUS was removed compared to 77% miscarriage when the IUS remained in the uterus. Studies on Paragard and birth defects have not been conducted.
8.2 Lactation
Risk Summary
No difference has been detected in concentration of copper in human milk before and after insertion of copper IUSs, including Paragard. There is no information on the effect of copper in a breastfed child or the effect on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Paragard and any potential adverse effects on the breastfed child from Paragard.
8.4 Pediatric Use
The safety and effectiveness of Paragard have been established in females of reproductive potential. Efficacy is expected to be the same for postmenarcheal females regardless of age.
Paragard is not indicated in females before menarche.
8.5 Geriatric Use
Paragard has not been studied in women over 65 years of age and is not indicated in this population.
4 CONTRAINDICATIONS
The use of Paragard is contraindicated when one or more of the following conditions exist:
- Pregnancy or suspicion of pregnancy [see Warnings and Precautions (5.1 , 5.2 ) and Use in Specific Populations (8.1 )]
- Abnormalities of the uterus resulting in distortion of the uterine cavity
- Acute pelvic inflammatory disease (PID) [see Warnings and Precautions (5.4 )]
- Postpartum endometritis or postabortal endometritis in the past 3 months [see Warnings and Precautions (5.4 )]
- Known or suspected uterine or cervical malignancy
- Uterine bleeding of unknown etiology
- Untreated acute cervicitis or vaginitis or other lower genital tract infection
- Conditions associated with increased susceptibility to pelvic infections [see Warnings and Precautions (5.4 )]
- Wilson’s disease [see Warnings and Precautions (5.9 )]
- A previously placed IUD or IUS that has not been removed
- Hypersensitivity to any component of Paragard including to copper or any of the trace elements present in the copper component of Paragard [see Adverse Reactions (6.2 ) and Description (11 )]
5 WARNINGS AND PRECAUTIONS
- Ectopic Pregnancy: Promptly evaluate women who become pregnant for ectopic pregnancy while using Paragard. (5.1 )
- Risks with Intrauterine Pregnancy : Increased risk of spontaneous abortion, septic abortion, premature delivery, sepsis, septic shock and death if pregnancy occurs. Remove Paragard if pregnancy occurs with Paragard in place. (5.2 )
- Sepsis: Group A streptococcal infection has been reported; strict aseptic technique is essential during insertion (5.3 )
- Pelvic Inflammatory Disease (PID) and Endometritis: Before using Paragard, consider the risks of PID and endometritis. Promptly assess and treat patients with signs and symptoms of PID. (5.4 )
- Embedment : Surgical removal may be necessary. (5.5 )
- Perforation : May reduce contraceptive effectiveness and require surgery. Risk is increased if inserted in lactating women and may be increased if inserted in women with fixed, retroverted uteri or noninvoluted uteri. (5.6 )
- Expulsion: Partial or complete expulsion may occur. Remove a partially expelled Paragard. (5.7 )
- Bleeding patterns: May be altered and result in heavier and longer bleeding with spotting. (5.9 )
- MRI Safety Information : Patients using Paragard can be safely scanned with MRI only under certain conditions. (5.10 )
Ectopic Pregnancy
Evaluate for possible ectopic pregnancy in any female who becomes pregnant while using Paragard because a pregnancy that occurs with Paragard in place is more likely to be ectopic than a pregnancy in the general population. However, because Paragard prevents most pregnancies, females who use Paragard have a lower risk of an ectopic pregnancy than sexually active females who do not use any contraception.
The incidence of ectopic pregnancy in the clinical trials with Paragard (which excluded females with a previous history of ectopic pregnancy) was approximately 0.06%. Ectopic pregnancy may require surgery and may result in loss of fertility.
Risks with Intrauterine Pregnancy
If intrauterine pregnancy occurs with Paragard in place and the strings are visible or can be retrieved from the cervical canal, remove Paragard because leaving it in place may increase the risk of spontaneous abortion and preterm labor. Removal of Paragard may also result in spontaneous abortion. In the event of an intrauterine pregnancy with Paragard, consider the following:
Septic Abortion
In females becoming pregnant with an intrauterine system (IUS), including Paragard in place, septic abortion, with septicemia, septic shock, and death, may occur [see Warnings and Precautions (5.3 )]. Septic abortion typically requires hospitalization and treatment with intravenous antibiotics. Septic abortion may result in spontaneous abortion or a medical indication for pregnancy termination. A hysterectomy may be required if severe infection of the uterus occurs, which will result in permanent infertility.
Continuation of Pregnancy
If a female becomes pregnant with Paragard in place and if Paragard cannot be removed or the female chooses not to have it removed, warn her that failure to remove Paragard increases the risk of miscarriage, sepsis, premature labor, and premature delivery. Prenatal care should include counseling about these risks and that she should report immediately any flu-like symptoms, fever, chills, cramping, pain, bleeding, vaginal discharge or leakage of fluid, or any other symptom that suggests complications of the pregnancy.
5.3 Sepsis
Severe infection or sepsis, including Group A Streptococcal Sepsis (GAS), have been reported following insertion of IUSs, including Paragard. In some cases, severe pain occurred within hours of insertion followed by sepsis within days. Because death from GAS is more likely if treatment is delayed, it is important to be aware of these rare but serious infections. Aseptic technique during insertion of Paragard is essential in order to minimize serious infections such as GAS [see Dosage and Administration (2.3 )].
5.4 Pelvic Inflammatory Disease and Endometritis
Insertion of Paragard is contraindicated in the presence of known or suspected Pelvic Inflammatory Disease (PID) or endometritis [see Contraindications (4 )]. IUSs, including Paragard, have been associated with an increased risk of PID, most likely due to organisms being introduced into the uterus during insertion. In the clinical trials with Paragard, the incidence of PID that resulted in the removal of Paragard was approximately 0.1% [see Clinical Studies (14 )].
Counsel women who receive Paragard to notify a healthcare provider if they have complaints of lower abdominal or pelvic pain, odorous discharge, unexplained bleeding, fever, or genital lesions or sores. In such circumstances, perform a pelvic examination promptly to evaluate for possible pelvic infection. Remove Paragard in cases of recurrent PID or endometritis, or if an acute pelvic infection is severe or does not respond to treatment.
PID can have serious consequences, such as tubal damage (leading to ectopic pregnancy or infertility), hysterectomy, sepsis, and death.
Females at Increased Risk for PID
PID or endometritis are often associated with a sexually transmitted infection (STI) and Paragard does not protect against STIs. The risk of PID or endometritis is greater for females who have multiple sexual partners, and also for females whose sexual partner(s) have multiple sexual partners. Females who have had PID or endometritis are at increased risk for a recurrence or re-infection. In particular, ascertain whether a female is at increased risk of infection (for example, leukemia, acquired immune deficiency syndrome (AIDS), intravenous drug abuse).
Asymptomatic PID
PID or endometritis may be asymptomatic but still result in tubal damage and its sequelae.
Treatment of PID or Endometritis in Patients Using Paragard
Remove Paragard in cases of recurrent endometritis or PID, or if an acute pelvic infection is severe or does not respond to treatment. Prophylactic antibiotics administered at the time of insertion do not appear to lower the incidence of PID.
Promptly assess and treat any female who develops signs or symptoms of PID. Perform appropriate testing for sexually transmitted infection and initiate antibiotic therapy promptly. Paragard does not need to be removed immediately. Reassess the patient in 48-72 hours. If no clinical improvement occurs, continue antibiotics and consider removal of Paragard. If the decision is to remove Paragard, start antibiotics prior to removal to avoid the potential risk for bacterial spread resulting from the removal procedure.
Actinomycosis
Actinomycosis has been associated with IUS use, including Paragard. Symptomatic women with known actinomycosis infection should have Paragard removed and receive antibiotics. Actinomycetes can be found in the genital tract cultures in healthy women without IUSs. The significance of actinomyces-like organisms on a Papanicolaou (PAP) smear in an asymptomatic IUS user is unknown, and this finding alone does not always require IUS removal and treatment. When possible, confirm a PAP smear diagnosis with cultures.
5.5 Embedment
Partial penetration or embedment of Paragard in the myometrium can make removal difficult. In some cases, surgical removal may be necessary. Breakage of an embedded Paragard during non-surgical removal has been reported [see Dosage and Administration (2.6 )].
5.6 Perforation
Partial or total perforation of the uterine wall or cervix may occur during insertions, although the perforation may not be detected until sometime later. Perforation may reduce contraceptive efficacy and result in pregnancy. The incidence of perforation during or following Paragard insertion in clinical trials was 0.2% (13 out of 5344).
Delayed detection or removal of Paragard in cases of perforation may result in migration outside the uterine cavity, adhesions, peritonitis, intestinal penetration, intestinal obstruction, abscesses and/or damage to adjacent organs.
A postmarketing safety study conducted in Europe (EURAS IUD) with IUSs, including copper IUSs, demonstrated an increased risk of perforation in lactating women. The risk of perforation may be increased if an IUS, such as Paragard, is inserted when the uterus is fixed, retroverted or not completely involuted during the postpartum period.
If perforation does occur, locate and remove Paragard promptly. Surgery may be required. Preoperative imaging followed by laparoscopy or laparotomy is often required to remove Paragard from the peritoneal cavity.
5.7 Expulsion
Partial or complete expulsion of Paragard has been reported, resulting in the loss of contraceptive protection. The incidence of expulsion in the clinical trials with Paragard was approximately 2.3%. Consider further diagnostic imaging, such as x-ray, to confirm expulsion if the IUS is not found in the uterus.
Paragard has been placed immediately after delivery, although the risk of expulsion may be increased when the uterus is not completely involuted at the time of insertion. Remove a partially expelled Paragard.
5.8 Wilson’s Disease
Paragard may exacerbate Wilson’s disease, a rare genetic disease affecting copper excretion; therefore, the use of Paragard is contraindicated in females of reproductive potential with Wilson’s disease [see Contraindications (4 )].
5.9 Bleeding Pattern Alterations
Paragard can alter the bleeding pattern and result in heavier and longer menstrual cycles with intermenstrual spotting.
In two clinical trials with Paragard® [see Adverse Reactions (6.1 )] , there were reports of oligomenorrhea and amenorrhea; however, a causal relationship between Paragard and these events could not be established. Menstrual changes were the most common medical reason for discontinuation of Paragard. Discontinuation rates for pain and bleeding combined were highest in the first year of use and diminished thereafter. The percentage of females who discontinued Paragard because of bleeding problems or pain during these studies ranged from 12% in the first year to 2% in Year 9. Females complaining of heavy vaginal bleeding should be evaluated and treated, and may need to discontinue Paragard [see Adverse Reactions (6.1 )] .
5.10 Magnetic Resonance Imaging (MRI) Safety Information
Non-clinical testing has demonstrated that Paragurad is MR Conditional. A patient with Paragard can be safely scanned in an MR system meeting the following conditions:
- Static magnetic field of 3.0 T or 1.5 T
- Maximum spatial field gradient of 4,000 gauss/cm (40 T/m)
- Maximum MR system reported, whole body averaged specific absorption rate (SAR) of 2 W/kg (Normal Operating Mode)
Under the scan conditions defined above, Paragard is expected to produce a maximum temperature rise of less than 0.5˚ C after 15 minutes of continuous scanning.
In non-clinical testing, the image artifact caused by the system extended less than 5 mm from the implant when imaged with a gradient echo pulse sequence and a 3.0 T MRI system.
5.11 Medical Diathermy
Medical equipment that contain high level of Radiofrequency (RF) energy such as diathermy may cause health effects (by heating tissue) in females with a metal-containing IUD including Paragard. Avoid using high medical RF transmitter devices in females with Paragard.
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling:
- Ectopic pregnancy [see Warnings and Precautions (5.1 )]
- Intrauterine pregnancy [see Warnings and Precautions (5.2 )]
- Septic abortion [see Warnings and Precautions (5.2 )]
- Group A Streptococcal Sepsis (GAS) [see Warnings and Precautions (5.3 )]
- Pelvic Inflammatory Disease and Endometritis [see Warnings and Precautions (5.4 )]
- Embedment [see Warnings and Precautions (5.5 )]
- Perforation [see Warnings and Precautions (5.6 )]
- Expulsion [see Warnings and Precautions (5.7 )]
- Bleeding Pattern Alterations [see Warnings and Precautions (5.9 )]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The data described below reflect exposure in two trials [see Clinical Studies (14 )].
- The WHO Study 79914 was a randomized, multicenter, multinational study of copper T IUSs, including Paragard in 1,396 women outside the US. In the WHO Study, 100% were parous and the mean age at enrollment was 29 years old.
- The US Composite Study was a meta-analysis that evaluated randomized, double-blind, comparative studies of copper T IUSs, including Paragard in 3,536 women in the US. In the US Composite Study, 64% were nulliparous, 49% were nulligravida, 68% were under age 25 at the time of enrollment (median age 23 years old).
Table 2 shows discontinuation rates from the two clinical studies by adverse reaction and year.
Table 2: Summary of Rates• (No. per 100 Subjects) by Year for Adverse Reactions Causing Discontinuation
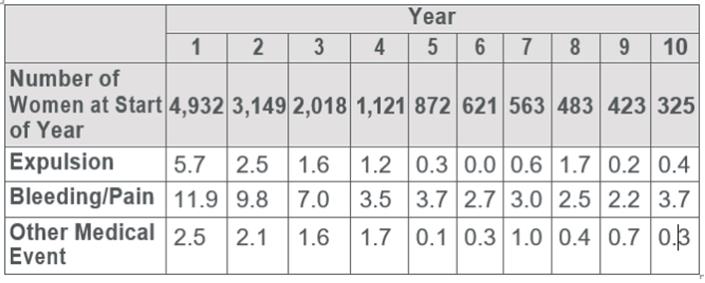
•Rates were calculated by weighting the annual rates by the number of subjects starting each year for each of the US Composite Study (3536 subjects) and the World Health Organization (1396 subjects) trials.
The following adverse reactions have also been observed: anemia, backache, dysmenorrhea, dyspareunia, complete or partial expulsion, prolonged menstrual flow, menstrual spotting, pain and cramping, and vaginitis.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Paragard. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal Disorders: abdominal distension, nausea
General Disorders and Administration Site Conditions: device breakage, pyrexia
Immune System Disorders : allergy to metals, hypersensitivity
Infections and Infestations: endometritis/uterine infection
Musculoskeletal and Connective Tissue Disorders : muscle spasms
Nervous System Disorders : dizziness
Reproductive System and Breast Disorders: amenorrhea
Skin and Subcutaneous Tissue Disorders: Stevens-Johnson syndrome
7 DRUG INTERACTIONS
No drug-drug interaction or drug-herbal supplement interaction studies have been conducted with Paragard.
11 DESCRIPTION
Paragard (intrauterine copper contraceptive) is a T-shaped intrauterine system (IUS), measuring 32 mm horizontally and 36 mm vertically, with a 3 mm diameter bulb at the tip of the vertical stem. (See Figure 1) [see Dosage and Administration (2.3 )]. A monofilament polyethylene thread is tied through the tip, resulting in two white threads, each at least 10.5 cm in length, to aid in detection and removal of the intrauterine system. The T-frame is made of polyethylene with barium sulfate to aid in detecting the intrauterine system under x-ray. Paragard also contains copper (approximately 176 mg of wire wrapped around the vertical stem and an approximately 68.7 mg collar placed on each side of the horizontal arm). The total exposed copper surface area is 380 ± 23 mm². One Paragard weighs less than one (1) gram. Paragard and its components are not made with natural rubber latex.
Paragard is packaged together with an insertion tube and solid white rod in a Tyvek® polyethylene pouch that is then sterilized. A moveable blue flange on the insertion tube aids in gauging the depth of insertion through the cervical canal and into the uterine cavity (See Figure 1) [see Dosage and Administration (2.3 )].
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Copper continuously released into the uterine cavity contributes to the contraceptive effectiveness of Paragard. Mechanism(s) by which copper enhances contraceptive efficacy include interference with sperm transport and fertilization of an egg, and possibly prevention of implantation.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Adequate long-term studies in animals to assess the carcinogenic potential of a copper-containing IUS have not been performed.
The genotoxic potential of a copper-containing IUS has not been evaluated.
14 CLINICAL STUDIES
The efficacy of Paragard for use in females of reproductive potential for prevention of pregnancy for up to 10 years was demonstrated in two trials:
- WHO Study 79914 was a randomized, multicenter, multinational study of copper T IUSs, including Paragard in 1,396 women outside the U.S. In the WHO study, 100% were parous and the mean age at enrollment was 29 years old.
- The U.S. Composite Study was a meta-analysis that evaluated several randomized, double-blind, comparative studies of copper T IUSs, including Paragard in 3,536 women in the US. In the US study, 64% were nulliparous, 49% were nulligravida; 68% were under age 25 at the time of enrollment (median age 23 years old).
The pregnancy rate in the two clinical studies with Paragard was less than 1 pregnancy per 100 women each year (see Table 3).
Table 3: Pregnancy Rates• (Number of Pregnancies per 100 Women) by Year in the WHO and U.S. Studies
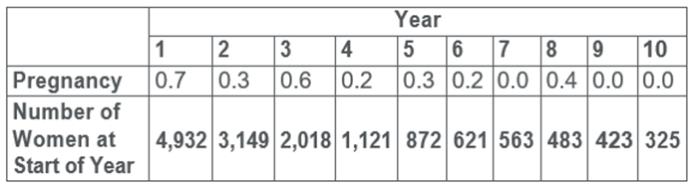
•Rates were calculated by weighting the annual rates by the number of subjects starting each year for each of the US Composite Study (3536 subjects) and the World Health Organization (1396 subjects) trials.
16 HOW SUPPLIED/STORAGE AND HANDLING
Paragard (intrauterine copper contraceptive) is available in cartons of 1 (one) sterile unit (NDC 59365-5128-1).
Each Paragard is white, T-shaped, and measures 32 mm horizontally and 36 mm vertically, with approximately 176 mg of copper wire wrapped around the vertical arm and an approximately 68.7 mg copper wire collar placed on each side of the horizontal arms, and with a monofilament polyethylene thread tied through the tip of the vertical arm [see Dosage and Administration (2.3 )]. The T-frame is made of polyethylene with barium sulfate. Each Paragard is packaged together with an insertion tube with blue flange and solid white rod in a Tyvek® polyethylene pouch.
Store at controlled room temperature: 59° to 86°F (15° to 30°C).
12.1 Mechanism of Action
Copper continuously released into the uterine cavity contributes to the contraceptive effectiveness of Paragard. Mechanism(s) by which copper enhances contraceptive efficacy include interference with sperm transport and fertilization of an egg, and possibly prevention of implantation.