Get your patient on Pifeltro (Doravirine)
Pifeltro prior authorization resources
Most recent state uniform prior authorization forms
Pifeltro patient education
Patient toolkit
Dosage & administration
DOSAGE AND ADMINISTRATION
Recommended Dosage
The recommended dosage regimen of PIFELTRO in adults and pediatric patients weighing at least 35 kg is one 100 mg tablet taken orally once daily with or without food [see Clinical Pharmacology (12.3) ] .
Dosage Adjustment with Rifabutin
If PIFELTRO is co-administered with rifabutin, increase PIFELTRO dosage to one tablet twice daily (approximately 12 hours apart) for the duration of rifabutin co-administration [see Drug Interactions (7.1) and Clinical Pharmacology (12.3) ].

By using PrescriberAI, you agree to the AI Terms of Use.
Pifeltro prescribing information
| Warnings and Precautions, Severe Skin Reactions (5.1 ) | 11/2024 |
INDICATIONS AND USAGE
PIFELTRO ® is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults and pediatric patients weighing at least 35 kg:
- with no prior antiretroviral treatment history; OR
- to replace the current antiretroviral regimen in those who are virologically-suppressed (HIV-1 RNA less than 50 copies per mL) on a stable antiretroviral regimen with no history of treatment failure and no known substitutions associated with resistance to doravirine [see Clinical Studies (14) ] .
DOSAGE AND ADMINISTRATION
Recommended Dosage
The recommended dosage regimen of PIFELTRO in adults and pediatric patients weighing at least 35 kg is one 100 mg tablet taken orally once daily with or without food [see Clinical Pharmacology (12.3) ] .
Dosage Adjustment with Rifabutin
If PIFELTRO is co-administered with rifabutin, increase PIFELTRO dosage to one tablet twice daily (approximately 12 hours apart) for the duration of rifabutin co-administration [see Drug Interactions (7.1) and Clinical Pharmacology (12.3) ].
DOSAGE FORMS AND STRENGTHS
PIFELTRO film-coated tablets are white, oval-shaped tablets, debossed with the corporate logo and 700 on one side and plain on the other side. Each tablet contains 100 mg doravirine.
USE IN SPECIFIC POPULATIONS
- Pediatrics: Not recommended for patients weighing less than 35 kg. (8.4 )
Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in individuals exposed to PIFELTRO during pregnancy. Healthcare providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry (APR) at 1-800-258-4263.
Risk Summary
No adequate human data are available to establish whether or not PIFELTRO poses a risk to pregnancy outcomes. In animal reproduction studies, no adverse developmental effects were observed when doravirine was administered at exposures ≥8 times the exposure in humans at the recommended human dose (RHD) of PIFELTRO ( see Data ).
The background rate of major birth defects is 2.7% in a U.S. reference population of the Metropolitan Atlanta Congenital Defects Program (MACDP). The rate of miscarriage is not reported in the APR. The estimated background rate of miscarriage in the clinically recognized pregnancies in the U.S. general population is 15-20%. Methodological limitations of the APR include the use of MACDP as the external comparator group. The MACDP population is not disease-specific, evaluates individuals and infants from the limited geographic area, and does not include outcomes for births that occurred at less than 20 weeks gestation.
Data
Animal Data
Doravirine was administered orally to pregnant rabbits (up to 300 mg/kg/day on gestation days (GD) 7 to 20) and rats (up to 450 mg/kg/day on GD 6 to 20 and separately from GD 6 to lactation/postpartum day 20). No significant toxicological effects on embryo-fetal (rats and rabbits) or pre/post-natal (rats) development were observed at exposures (AUC) approximately 9 times (rats) and 8 times (rabbits) the exposure in humans at the RHD. Doravirine was transferred to the fetus through the placenta in embryo-fetal studies, with fetal plasma concentrations of up to 40% (rabbits) and 52% (rats) that of maternal concentrations observed on GD 20.
Lactation
Risk Summary
It is unknown whether doravirine is present in human milk, affects human milk production, or has effects on the breastfed infant. Doravirine is present in the milk of lactating rats ( see Data ). Potential risks of breastfeeding include: (1) HIV-1 transmission (in HIV-1-negative infants), (2) developing viral resistance (in HIV-1-positive infants), and (3) serious adverse reactions in a breastfed infant similar to those seen in adults.
Data
Doravirine was excreted into the milk of lactating rats following oral administration (450 mg/kg/day) from GD 6 to lactation day 14, with milk concentrations approximately 1.5 times that of maternal plasma concentrations observed 2 hours post dose on lactation day 14.
Pediatric Use
The safety and efficacy of PIFELTRO for the treatment of HIV-1 infection have been established in pediatric patients weighing at least 35 kg [see Indications and Usage (1) and Dosage and Administration (2.1) ].
Use of PIFELTRO in this group is supported by evidence from adequate and well-controlled trials in adults and an open-label trial in virologically-suppressed or treatment-naïve pediatric participants 12 to less than 18 years of age. The safety, efficacy, and exposure of doravirine in these pediatric participants were similar to that in adults [see Adverse Reactions (6.1) , Clinical Pharmacology (12.3) , and Clinical Studies (14.3) ].
Safety and efficacy of PIFELTRO in pediatric patients weighing less than 35 kg have not been established.
Geriatric Use
Clinical trials of PIFELTRO did not include sufficient numbers of participants aged 65 years and over to determine whether they respond differently from younger participants. In general, caution should be exercised in the administration of PIFELTRO in elderly patients, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy [see Clinical Pharmacology (12.3) ] .
Renal Impairment
No dosage adjustment of PIFELTRO is required in patients with mild, moderate, or severe renal impairment. PIFELTRO has not been adequately studied in patients with end-stage renal disease and has not been studied in dialysis patients [see Clinical Pharmacology (12.3) ] .
Hepatic Impairment
No dosage adjustment of PIFELTRO is required in patients with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment. PIFELTRO has not been studied in patients with severe hepatic impairment (Child-Pugh Class C) [see Clinical Pharmacology (12.3) ] .
CONTRAINDICATIONS
PIFELTRO is contraindicated when co-administered with drugs that are strong cytochrome P450 (CYP)3A enzyme inducers as significant decreases in doravirine plasma concentrations may occur, which may decrease the effectiveness of PIFELTRO [see Warnings and Precautions (5.2) , Drug Interactions (7.1) , and Clinical Pharmacology (12.3) ] . These drugs include, but are not limited to, the following:
- the anticonvulsants carbamazepine, oxcarbazepine, phenobarbital, phenytoin
- the androgen receptor inhibitor enzalutamide
- the antimycobacterials rifampin, rifapentine
- the cytotoxic agent mitotane
- St. John's wort ( Hypericum perforatum)
WARNINGS AND PRECAUTIONS
- Severe skin reactions, including Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN), have been reported during the postmarketing experience with doravirine-containing regimens. Discontinue PIFELTRO, and other medications known to be associated with severe skin reactions, immediately if a painful rash with mucosal involvement or a progressive severe rash develops, and closely monitor clinical status. (5.1 )
- Monitor for Immune Reconstitution Syndrome. (5.3 )
5.1 Severe Skin Reactions
Severe skin reactions, including Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN), have been reported during the postmarketing experience with doravirine-containing regimens [see Adverse Reactions (6.2) ]. Discontinue PIFELTRO, and other medications known to be associated with severe skin reactions, immediately if a painful rash with mucosal involvement or a progressive severe rash develops. Clinical status should be closely monitored, and appropriate therapy should be initiated.
Risk of Adverse Reactions or Loss of Virologic Response Due to Drug Interactions
The concomitant use of PIFELTRO and certain other drugs may result in known or potentially significant drug interactions, some of which may lead to loss of therapeutic effect of PIFELTRO and possible development of resistance [see Dosage and Administration (2.2) , Contraindications (4) and Drug Interactions (7.1) ].
See Table 6 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations. Consider the potential for drug interactions prior to and during PIFELTRO therapy, review concomitant medications during PIFELTRO therapy, and monitor for adverse reactions.
Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia (PCP), or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves' disease, polymyositis, Guillain-Barré syndrome, and autoimmune hepatitis) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable and can occur many months after initiation of treatment.
ADVERSE REACTIONS
The following adverse reactions are discussed in other sections of the labeling:
- Immune Reconstitution Syndrome [see Warnings and Precautions (5.3) ]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions in Adults with No Antiretroviral Treatment History
The safety assessment of PIFELTRO used in combination with other antiretroviral agents is based on Week 96 data from two Phase 3, randomized, international, multicenter, double-blind, active-controlled trials (DRIVE-FORWARD (Protocol 018) and DRIVE-AHEAD (Protocol 021)).
In DRIVE-FORWARD, 766 adult participants received either PIFELTRO 100 mg (n=383) or darunavir 800 mg + ritonavir 100 mg (DRV+r) (n=383) once daily, each in combination with emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) or abacavir/lamivudine (ABC/3TC). By Week 96, 2% in the PIFELTRO group and 3% in the DRV+r group had adverse events leading to discontinuation of study medication.
In DRIVE-AHEAD, 728 adult participants received either DELSTRIGO [doravirine (DOR)/3TC/TDF] (n=364) or efavirenz (EFV)/FTC/TDF once daily (n=364). By Week 96, 3% in the DELSTRIGO group and 7% in the EFV/FTC/TDF group had adverse events leading to discontinuation of study medication.
Adverse reactions reported in greater than or equal to 5% of participants in any treatment group in DRIVE-FORWARD and DRIVE-AHEAD are presented in Table 1.
| DRIVE-FORWARD | DRIVE-AHEAD | |||
|---|---|---|---|---|
| PIFELTRO+2 NRTIs NRTI = nucleoside reverse transcriptase inhibitor. Once Daily N=383 | DRV+r+2 NRTIs Once Daily N=383 | DELSTRIGO Once Daily N=364 | EFV/FTC/TDF Once Daily N=364 | |
| NRTIs = FTC/TDF or ABC/3TC. Fatigue: includes fatigue, asthenia, malaise Abdominal Pain: includes abdominal discomfort, abdominal pain, abdominal pain lower, abdominal pain upper, epigastric discomfort Rash: includes rash, rash erythematous, rash generalized, rash macular, rash maculo-papular, rash papular, rash pruritic, rash pustular | ||||
| Nausea | 7% | 8% | 5% | 7% |
| Headache | 6% | 3% | 4% | 5% |
| Fatigue | 6% | 3% | 4% | 4% |
| Diarrhea | 6% | 13% | 4% | 6% |
| Abdominal Pain | 5% | 2% | 1% | 2% |
| Dizziness | 3% | 2% | 7% | 32% |
| Rash | 2% | 3% | 2% | 12% |
| Abnormal Dreams | 1% | <1% | 5% | 10% |
| Insomnia | 1% | 2% | 4% | 5% |
| Somnolence | 0% | <1% | 3% | 7% |
The majority (77%) of adverse reactions associated with doravirine occurred at severity Grade 1 (mild).
Neuropsychiatric Adverse Events
For DRIVE-AHEAD, the analysis of participants with neuropsychiatric adverse events by Week 48 is presented in Table 2. The proportion of participants who reported one or more neuropsychiatric adverse events was 24% and 57% in the DELSTRIGO and EFV/FTC/TDF groups, respectively.
A statistically significantly lower proportion of DELSTRIGO-treated participants compared to EFV/FTC/TDF-treated participants reported neuropsychiatric adverse events by Week 48 in the three pre-specified categories of dizziness, sleep disorders and disturbances, and altered sensorium.
| DELSTRIGO Once Daily N=364 | EFV/FTC/TDF Once Daily N=364 | Treatment Difference DELSTRIGO - EFV/FTC/TDF Estimate (95% CI) The 95% CIs were calculated using Miettinen and Nurminen's method. Categories pre-specified for statistical testing were dizziness (p <0.001), sleep disorders and disturbances (p <0.001), and altered sensorium (p=0.033). | |
|---|---|---|---|
| Sleep disorders and disturbances Predefined using MedDRA preferred terms, including: abnormal dreams, hyposomnia, initial insomnia, insomnia, nightmare, sleep disorder, somnambulism. | 12% | 26% | -13.5 (-19.1, -7.9) |
| Dizziness | 9% | 37% | -28.3 (-34.0, -22.5) |
| Altered sensorium Predefined using MedDRA preferred terms, including: altered state of consciousness, lethargy, somnolence, syncope. | 4% | 8% | -3.8 (-7.6, -0.3) |
Neuropsychiatric adverse events in the pre-defined category of depression and suicide/self-injury were reported in 4% and 7% of participants, in the DELSTRIGO and EFV/FTC/TDF groups, respectively.
In DRIVE-AHEAD through 48 weeks of treatment, the majority of participants who reported neuropsychiatric adverse events reported events that were mild to moderate in severity (97% [83/86] and 96% [198/207], in the DELSTRIGO and EFV/FTC/TDF groups, respectively) and the majority of participants reported these events in the first 4 weeks of treatment (72% [62/86] in the DELSTRIGO group and 86% [177/207] in the EFV/FTC/TDF group).
Neuropsychiatric adverse events led to treatment discontinuation in 1% (2/364) and 1% (5/364) of participants in the DELSTRIGO and EFV/FTC/TDF groups, respectively. The proportion of participants who reported neuropsychiatric adverse events through Week 4 was 17% (62/364) in the DELSTRIGO group and 49% (177/364) in the EFV/FTC/TDF group. At Week 48, the prevalence of neuropsychiatric adverse events was 12% (44/364) in the DELSTRIGO group and 22% (81/364) in the EFV/FTC/TDF group. At Week 96, the prevalence of neuropsychiatric adverse events was 13% (47/364) in the DELSTRIGO group and 23% (82/364) in the EFV/FTC/TDF group.
Laboratory Abnormalities
The percentages of participants with selected laboratory abnormalities (that represent a worsening from baseline) who were treated with PIFELTRO or DRV+r in DRIVE-FORWARD, or DELSTRIGO or EFV/FTC/TDF in DRIVE-AHEAD are presented in Table 3.
| DRIVE-FORWARD | DRIVE-AHEAD | |||
|---|---|---|---|---|
| Laboratory Parameter Preferred Term (Unit)/Limit | PIFELTRO+2 NRTIs Once Daily N=383 | DRV+r+2 NRTIs Once Daily N=383 | DELSTRIGO Once Daily N=364 | EFV/FTC/TDF Once Daily N=364 |
| Blood Chemistry | ||||
| Each participant is only counted once per parameter at the highest toxicity grade. Only participants with a baseline value and at least one on-treatment value for a given laboratory parameter are included. ULN = Upper limit of normal range. Note: NRTIs = FTC/TDF or ABC/3TC. | ||||
| Total bilirubin (mg/dL) | ||||
| 1.1 - < 1.6 × ULN | 6% | 2% | 5% | 0% |
| 1.6 - <2.6 × ULN | 2% | <1% | 2% | 0% |
| ≥2.6 × ULN | <1% | 0% | 1% | <1% |
| Creatinine (mg/dL) | ||||
| >1.3 - 1.8 × ULN or Increase of >0.3 mg/dL above baseline | 4% | 6% | 3% | 2% |
| >1.8 × ULN or Increase of ≥1.5 × above baseline | 4% | 4% | 3% | 2% |
| Aspartate aminotransferase (IU/L) | ||||
| 2.5 - <5.0 × ULN | 5% | 4% | 3% | 3% |
| ≥5.0 × ULN | 2% | 2% | 1% | 4% |
| Alanine aminotransferase (IU/L) | ||||
| 2.5 - <5.0 × ULN | 4% | 2% | 4% | 4% |
| ≥5.0 × ULN | 2% | 3% | 1% | 3% |
| Alkaline phosphatase (IU/L) | ||||
| 2.5 - <5.0 × ULN | <1% | 1% | <1% | 1% |
| ≥5.0 × ULN | 0% | <1% | 0% | <1% |
| Lipase | ||||
| 1.5 - <3.0 × ULN | 7% | 6% | 6% | 4% |
| ≥3.0 × ULN | 3% | 4% | 2% | 3% |
| Creatine kinase (IU/L) | ||||
| 6.0 - <10.0 × ULN | 3% | 3% | 3% | 3% |
| ≥10.0 × ULN | 5% | 6% | 4% | 6% |
| Cholesterol, fasted (mg/dL) | ||||
| ≥300 mg/dL | 0% | 1% | 1% | <1% |
| LDL cholesterol, fasted (mg/dL) | ||||
| ≥190 mg/dL | <1% | 4% | <1% | 2% |
| Triglycerides, fasted (mg/dL) | ||||
| >500 mg/dL | 1% | 2% | 1% | 3% |
Change in Lipids from Baseline
For DRIVE-FORWARD and DRIVE-AHEAD, changes from baseline at Week 48 in LDL-cholesterol, non-HDL-cholesterol, total cholesterol, triglycerides, and HDL-cholesterol are shown in Table 4. Changes from baseline at Week 96 were similar to those seen at Week 48.
The LDL and non-HDL comparisons were pre-specified and are summarized in Table 4. The differences were statistically significant, showing superiority for doravirine for both parameters. The clinical benefit of these findings has not been demonstrated.
| Participants on lipid-lowering agents at baseline were excluded from these analyses (in DRIVE-FORWARD: PIFELTRO n=12 and DRV+r n=14; in DRIVE-AHEAD: DELSTRIGO n=15 and EFV/FTC/TDF n=10). Participants initiating a lipid-lowering agent post-baseline had their last fasted on-treatment value (prior to starting the agent) carried forward (in DRIVE-FORWARD: PIFELTRO n=6 and DRV+r n=4; in DRIVE-AHEAD: DELSTRIGO n=3 and EFV/FTC/TDF n=8). | |||||
| DRIVE-FORWARD | |||||
| PIFELTRO+2 NRTIs Once Daily N=320 | DRV+r+2 NRTIs Once Daily N=311 | ||||
| Laboratory Parameter Preferred Term | Baseline | Change | Baseline | Change | Difference Estimates (95% CI) |
| LDL-Cholesterol (mg/dL) p-values for the pre-specified hypothesis testing for treatment difference were <0.0001 in both DRIVE-FORWARD and DRIVE-AHEAD. | 91.4 | -4.6 | 92.3 | 9.5 | -14.4 (-18.0, -10.8) |
| Non-HDL Cholesterol (mg/dL) | 113.6 | -5.4 | 114.5 | 13.7 | -19.4 (-23.4, -15.4) |
| Total Cholesterol (mg/dL) Not pre-specified for hypothesis testing. | 157.2 | -1.4 | 157.8 | 18.0 | - |
| Triglycerides (mg/dL) | 111.0 | -3.1 | 113.7 | 24.5 | - |
| HDL-Cholesterol (mg/dL) | 43.6 | 4.0 | 43.3 | 4.3 | - |
| DRIVE-AHEAD | |||||
| DELSTRIGO Once Daily N=320 | EFV/FTC/TDF Once Daily N=307 | ||||
| Laboratory Parameter Preferred Term | Baseline | Change | Baseline | Change | Difference Estimates (95% CI) |
| LDL-Cholesterol (mg/dL) | 91.7 | -2.1 | 91.3 | 8.3 | -10.2 (-13.8, -6.7) |
| Non-HDL Cholesterol (mg/dL) | 114.7 | -4.1 | 115.3 | 12.7 | -16.9 (-20.8, -13.0) |
| Total Cholesterol (mg/dL) | 156.8 | -2.2 | 156.8 | 21.1 | - |
| Triglycerides (mg/dL) | 118.7 | -12.0 | 122.6 | 21.6 | - |
| HDL-Cholesterol (mg/dL) | 42.1 | 1.8 | 41.6 | 8.4 | - |
Adverse Reactions in Virologically-Suppressed Adults
The safety of DELSTRIGO in virologically-suppressed adults was based on Week 48 data from 670 participants in the DRIVE-SHIFT trial (Protocol 024), a randomized, international, multicenter, open-label trial in which virologically-suppressed participants were switched from a baseline regimen consisting of two NRTIs in combination with a protease inhibitor (PI) plus either ritonavir or cobicistat, or elvitegravir plus cobicistat, or a non-nucleoside reverse transcriptase inhibitor (NNRTI) to DELSTRIGO. Overall, the safety profile in virologically-suppressed adult participants was similar to that in participants with no antiretroviral treatment history.
Laboratory Abnormalities
Serum ALT and AST Elevations: In the DRIVE-SHIFT trial, 22% and 16% of participants in the immediate switch group experienced ALT and AST elevations greater than 1.25 × ULN, respectively, through 48 weeks on DELSTRIGO. For these ALT and AST elevations, no apparent patterns with regard to time to onset relative to switch were observed. One percent of participants had ALT or AST elevations greater than 5 × ULN through 48 weeks on DELSTRIGO. The ALT and AST elevations were generally asymptomatic and not associated with bilirubin elevations. In comparison, 4% and 4% of participants in the delayed switch group experienced ALT and AST elevations of greater than 1.25 × ULN through 24 weeks on their baseline regimen.
Change in Lipids from Baseline
Changes from baseline at Week 24 in LDL-cholesterol, non-HDL-cholesterol, total cholesterol, triglycerides, and HDL-cholesterol in participants on a PI plus ritonavir-based regimen at baseline are shown in Table 5. The LDL and non-HDL comparisons were pre-specified, and the differences were statistically significant, showing superiority for an immediate switch to DELSTRIGO for both parameters. The clinical benefit of these findings has not been demonstrated.
| Laboratory Parameter Preferred Term | DELSTRIGO (Week 0-24) Once Daily N=244 | PI+ritonavir (Week 0-24) Once Daily N=124 | Difference Estimates | ||
|---|---|---|---|---|---|
| Baseline | Change | Baseline | Change | Difference (95% CI) | |
| Participants on lipid-lowering agents at baseline were excluded from these analyses (DELSTRIGO n=26 and PI+ritonavir n=13). | |||||
| Participants initiating a lipid-lowering agent post-baseline had their last fasted on-treatment value (prior to starting the agent) carried forward (DELSTRIGO n=4 and PI+ritonavir n=2). | |||||
| LDL-Cholesterol (mg/dL) p-value for the pre-specified hypothesis testing for treatment difference was <0.0001. | 108.7 | -16.3 | 110.5 | -2.6 | -14.5 (-18.9, -10.1) |
| Non-HDL Cholesterol (mg/dL) | 138.6 | -24.8 | 138.8 | -2.1 | -22.8 (-27.9, -17.7) |
| Total Cholesterol (mg/dL) Not pre-specified for hypothesis testing. | 188.5 | -26.1 | 187.4 | -0.2 | - |
| Triglycerides (mg/dL) | 153.1 | -44.4 | 151.4 | -0.4 | - |
| HDL-Cholesterol (mg/dL) | 50.0 | -1.3 | 48.5 | 1.9 | - |
Adverse Reactions in Pediatric Participants
The safety of doravirine as a component of DELSTRIGO was evaluated in 45 virologically-suppressed or treatment-naïve pediatric participants 12 to less than 18 years of age living with HIV through Week 24 in an open-label trial (IMPAACT 2014 (Protocol 027)) [see Clinical Studies (14.3) ] . The safety profile in pediatric participants was similar to that in adults. There were no serious or Grade 3 or 4 adverse reactions. No participants discontinued due to an adverse event.
Postmarketing Experience
The following adverse reactions have been identified during postmarketing experience in patients receiving doravirine-containing regimens. Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and Subcutaneous Tissue Disorders: Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN)
Hepatobiliary Disorders : hepatitis
Investigations : hepatic enzyme increased
DRUG INTERACTIONS
Consult the full prescribing information prior to and during treatment for important potential drug-drug interactions. (4 , 5.2 , 7 )
Effect of Other Drugs on PIFELTRO
Co-administration of PIFELTRO with a CYP3A inducer decreases doravirine plasma concentrations, which may reduce PIFELTRO efficacy [see Contraindications (4) , Warnings and Precautions (5.2) , and Clinical Pharmacology (12.3) ] . Co-administration of PIFELTRO and drugs that are inhibitors of CYP3A may result in increased plasma concentrations of doravirine.
Table 6 shows significant drug interactions with PIFELTRO.
| Concomitant Drug Class: Drug Name | Effect on Concentration | Clinical Comment |
|---|---|---|
| ↑ = increase, ↓ = decrease All other drug-drug interactions shown are anticipated based on the known metabolic and elimination pathways. | ||
| Androgen Receptors | ||
| enzalutamide | ↓ doravirine | Co-administration is contraindicated with enzalutamide. At least a 4-week cessation period is recommended prior to initiation of PIFELTRO. |
| Anticonvulsants | ||
| carbamazepine oxcarbazepine phenobarbital phenytoin | ↓ doravirine | Co-administration is contraindicated with these anticonvulsants. At least a 4-week cessation period is recommended prior to initiation of PIFELTRO. |
| Antimycobacterials | ||
| rifampin The interaction between PIFELTRO and the concomitant drug was evaluated in a clinical study. rifapentine | ↓ doravirine | Co-administration is contraindicated with rifampin or rifapentine . At least a 4-week cessation period is recommended prior to initiation of PIFELTRO. |
| rifabutin | ↓ doravirine | Increase PIFELTRO dosage to one tablet twice daily when co-administered with rifabutin [see Dosage and Administration (2.2) ] . |
| Cytotoxic Agents | ||
| mitotane | ↓ doravirine | Co-administration is contraindicated with mitotane. At least a 4-week cessation period is recommended prior to initiation of PIFELTRO. |
| HIV Antiviral Agents | ||
| efavirenz etravirine nevirapine | ↓ doravirine | Use with efavirenz, etravirine, or nevirapine is not recommended. |
| Herbal Products | ||
| St. John's wort | ↓ doravirine | Co-administration is contraindicated with St. John's wort. At least a 4-week cessation period is recommended prior to initiation of PIFELTRO. |
No clinically significant changes in concentration were observed for doravirine when co-administered with the following agents: dolutegravir, TDF, lamivudine, elbasvir and grazoprevir, ledipasvir and sofosbuvir, ritonavir, ketoconazole, aluminum hydroxide/magnesium hydroxide/simethicone containing antacid, pantoprazole, and methadone [see Clinical Pharmacology (12.3) ].
Effect of PIFELTRO on Other Drugs
No clinically significant changes in concentration were observed for the following agents when co-administered with doravirine: dolutegravir, lamivudine, TDF, elbasvir and grazoprevir, ledipasvir and sofosbuvir, atorvastatin, an oral contraceptive containing ethinyl estradiol and levonorgestrel, metformin, methadone, and midazolam [see Clinical Pharmacology (12.3) ] .
DESCRIPTION
PIFELTRO is a film-coated tablet containing doravirine for oral administration.
Doravirine is an HIV-1 non-nucleoside reverse transcriptase inhibitor (NNRTI).
Each tablet contains 100 mg of doravirine as the active ingredient. The tablets include the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose acetate succinate, lactose monohydrate, magnesium stearate, and microcrystalline cellulose. The tablets are film coated with a coating material containing the following inactive ingredients: hypromellose, lactose monohydrate, titanium dioxide, and triacetin. The coated tablets are polished with carnauba wax.
The chemical name for doravirine is 3-chloro-5-[[1-[(4,5-dihydro-4-methyl-5-oxo-1 H -1,2,4-triazol-3-yl)methyl]-1,2-dihydro-2-oxo-4-(trifluoromethyl)-3-pyridinyl]oxy]benzonitrile.
It has a molecular formula of C 17 H 11 ClF 3 N 5 O 3 and a molecular weight of 425.75.
It has the following structural formula:
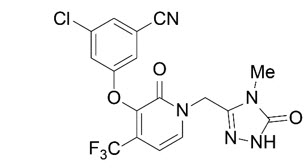
Doravirine is practically insoluble in water.
CLINICAL PHARMACOLOGY
Mechanism of Action
Doravirine is an antiretroviral drug [see Microbiology (12.4) ].
Pharmacodynamics
In a Phase 2 trial evaluating doravirine over a dose range of 0.25 to 2 times the recommended dose of PIFELTRO, (in combination with FTC/TDF) in participants living with HIV with no antiretroviral treatment history, no exposure-response relationship for efficacy was identified for doravirine.
Cardiac Electrophysiology
At a doravirine dose of 1200 mg, which provides approximately 4 times the peak concentration observed following the recommended dose of PIFELTRO, doravirine does not prolong the QT interval to any clinically relevant extent.
Pharmacokinetics
Doravirine pharmacokinetics are similar in healthy participants and participants living with HIV. Doravirine pharmacokinetics are provided in Table 7.
| Parameter | Doravirine |
|---|---|
| Abbreviations: AUC=area under the time concentration curve; C max =maximum concentration; C 24 =concentration at 24 hours; T max time to C max ; V dss = volume of distribution at steady state, t 1/2 =elimination half-life; CL/F=apparent clearance; CL renal =apparent renal clearance | |
| General | |
| Steady State Exposure Doravirine 100 mg once daily to participants living with HIV , Presented as geometric mean (%CV: geometric coefficient of variation) | |
| AUC 0-24 (mcg∙h/mL) | 16.1 (29) |
| C max (mcg/mL) | 0.962 (19) |
| C 24 (mcg/mL) | 0.396 (63) |
| Time to Steady State (Days) | 2 |
| Accumulation Ratio | 1.2 to 1.4 |
| Absorption | |
| Absolute Bioavailability | 64% |
| T max (h) | 2 |
| Effect of Food Geometric mean ratio [high-fat meal/fasting] and (90% confidence interval) for PK parameters. High fat meal is approximately 1,000 kcal, 50% fat. The effect of food is not clinically relevant. | |
| AUC Ratio | 1.16 (1.06, 1.26) |
| C max Ratio | 1.03 (0.89, 1.19) |
| C 24 Ratio | 1.36 (1.19, 1.55) |
| Distribution | |
| V dss (L) Based on IV dose | 60.5 |
| Plasma Protein Binding | 76% |
| Elimination | |
| t 1/2 (h) | 15 |
| CL/F (mL/min) | 106 (35.2) |
| CL renal (mL/min) | 9.3 (18.6) |
| Metabolism | |
| Primary Pathway(s) | CYP3A |
| Excretion | |
| Major Route of Elimination | Metabolism |
| Urine (unchanged) | 6% |
| Biliary/Fecal (unchanged) | Minor |
Specific Populations
In adults, no clinically significant difference on the pharmacokinetics of doravirine were observed based on age (18 to 78 years of age), sex, and race/ethnicity, mild to severe renal impairment (creatinine clearance (CLcr) >15 mL/min, estimated by Cockcroft-Gault), or moderate hepatic impairment (Child-Pugh B). The pharmacokinetics of doravirine in patients with end-stage renal disease or undergoing dialysis, or severe hepatic impairment (Child-Pugh C) is unknown.
Patients with Renal Impairment
In a study comparing 8 participants with severe renal impairment to 8 participants without renal impairment, the single dose exposure of doravirine was 43% higher in participants with severe renal impairment. In a population pharmacokinetic analysis, renal function did not have a clinically relevant effect on doravirine pharmacokinetics. Doravirine has not been studied in patients with end-stage renal disease or in patients undergoing dialysis [see Use in Specific Populations (8.6) ] .
Patients with Hepatic Impairment
No clinically significant difference in the pharmacokinetics of doravirine was observed in participants with moderate hepatic impairment (Child-Pugh score B) compared to participants without hepatic impairment. Doravirine has not been studied in participants with severe hepatic impairment (Child-Pugh score C) [see Use in Specific Populations (8.7) ] .
Pediatric Patients
Mean doravirine exposures were similar in 54 pediatric participants aged 12 to less than 18 years and weighing at least 35 kg who received doravirine or DELSTRIGO in IMPAACT 2014 (Protocol 027) relative to adults following administration of doravirine or DELSTRIGO (Table 8). For pediatric participants weighing ≥ 35 kg and < 45 kg who received doravirine 100 mg or DELSTRIGO, the population pharmacokinetic model-predicted mean C 24 of doravirine was comparable to that achieved in adults, whereas mean AUC 0-24 and C max of doravirine were 25% and 36% higher than adult values, respectively. However, the predicted AUC 0-24 and C max increases are not considered clinically significant.
| Parameter Presented as geometric mean (%CV: geometric coefficient of variation) | Doravirine From population PK analysis (n=53 weighing ≥45 kg, n=1 weighing ≥35 kg to <45 kg) |
|---|---|
| Abbreviations: AUC=area under the time concentration curve; C max =maximum concentration; C 24 =concentration at 24 hours | |
| AUC 0-24 (mcg∙h/mL) | 16.4 (24) |
| C max (mcg/mL) | 1.03 (16) |
| C 24 (mcg/mL) | 0.379 (42) |
Drug Interaction Studies
Doravirine is primarily metabolized by CYP3A, and drugs that induce or inhibit CYP3A may affect the clearance of doravirine. Co-administration of doravirine and drugs that induce CYP3A may result in decreased plasma concentrations of doravirine. Co-administration of doravirine and drugs that inhibit CYP3A may result in increased plasma concentrations of doravirine.
Doravirine is not likely to have a clinically relevant effect on the exposure of medicinal products metabolized by CYP enzymes. Doravirine did not inhibit major drug metabolizing enzymes in vitro , including CYPs 1A2, 2B6, 2C8, 2C9, 2C19, 2D6, 3A4, and UGT1A1 and is not likely to be an inducer of CYP1A2, 2B6, or 3A4. Based on in vitro assays, doravirine is not likely to be an inhibitor of OATP1B1, OATP1B3, P-glycoprotein, BSEP, OAT1, OAT3, OCT2, MATE1, and MATE2K. Drug interaction studies were performed with doravirine and other drugs likely to be co-administered or commonly used as probes for pharmacokinetic interactions. The effects of co-administration with other drugs on the exposure (C max , AUC, and C 24 ) of doravirine are summarized in Table 9. A single doravirine 100 mg dose was administered in these studies unless otherwise noted.
| Co-administered Drug | Regimen of Co-administered Drug | N | Geometric Mean Ratio (90% CI) of Doravirine Pharmacokinetics with/without Co-administered Drug (No Effect=1.00) | ||
|---|---|---|---|---|---|
| AUC AUC 0-∞ for single-dose, AUC 0-24 for once daily. | C max | C 24 | |||
| CI = confidence interval; QD = once daily; BID = twice daily | |||||
| Azole Antifungal Agents | |||||
| ketoconazole Changes in doravirine pharmacokinetic values are not clinically relevant. | 400 mg QD | 10 | 3.06 (2.85, 3.29) | 1.25 (1.05, 1.49) | 2.75 (2.54, 2.98) |
| Antimycobacterials | |||||
| rifampin | 600 mg QD | 10 | 0.12 (0.10, 0.15) | 0.43 (0.35, 0.52) | 0.03 (0.02, 0.04) |
| rifabutin | 300 mg QD | 12 | 0.50 (0.45, 0.55) | 0.99 (0.85, 1.15) | 0.32 (0.28, 0.35) |
| 300 mg QD Doravirine 100 mg BID resulted in similar pharmacokinetic values when compared to 100 mg QD without rifabutin. | 15 | 1.03 (0.94, 1.14) | 0.97 (0.87, 1.08) | 0.98 (0.88, 1.10) | |
| HIV Antiviral Agents | |||||
| ritonavir , A single doravirine 50 mg dose (0.5 times the recommended approved dose) was administered. | 100 mg BID | 8 | 3.54 (3.04, 4.11) | 1.31 (1.17, 1.46) | 2.91 (2.33, 3.62) |
| efavirenz | 600 mg QD The first day following the cessation of efavirenz therapy and initiation of doravirine 100 mg QD. | 17 | 0.38 (0.33, 0.45) | 0.65 (0.58, 0.73) | 0.15 (0.10, 0.23) |
| 600 mg QD 14 days following the cessation of efavirenz therapy and initiation of doravirine 100 mg QD. | 17 | 0.68 (0.58, 0.80) | 0.86 (0.77, 0.97) | 0.50 (0.39, 0.64) | |
Based on drug interaction studies conducted with doravirine, no clinically significant drug interactions have been observed following the co-administration of doravirine and the following drugs: dolutegravir, ritonavir, TDF, lamivudine, elbasvir and grazoprevir, ledipasvir and sofosbuvir, ketoconazole, aluminum hydroxide/magnesium hydroxide/simethicone containing antacid, pantoprazole, atorvastatin, an oral contraceptive containing ethinyl estradiol and levonorgestrel, metformin, methadone, and midazolam.
Microbiology
Mechanism of Action
Doravirine is a pyridinone non-nucleoside reverse transcriptase inhibitor of HIV-1 and inhibits HIV-1 replication by non-competitive inhibition of HIV-1 reverse transcriptase (RT). The inhibitory concentration at 50% (IC 50 ) of doravirine for RNA-dependent DNA polymerization of recombinant wild-type HIV-1 RT in a biochemical assay was 12.2±2.0 nM (n=3). Doravirine does not inhibit the human cellular DNA polymerases α, β, and mitochondrial DNA polymerase γ.
Antiviral Activity in Cell Culture
Doravirine exhibited an EC 50 value of 12.0±4.4 nM against wild-type laboratory strains of HIV-1 when tested in the presence of 100% normal human serum (NHS) using MT4-GFP reporter cells and a median EC 50 value for HIV-1 subtype B primary isolates (n=118) of 4.1 nM (range: 1.0 nM-16.0 nM). Doravirine demonstrated antiviral activity against a broad panel of primary HIV-1 isolates (A, A1, AE, AG, B, BF, C, D, G, H) with EC 50 values ranging from 1.2 nM to 10.0 nM.
Antiviral Activity in Combination with other HIV Antiviral Agents
The antiviral activity of doravirine in cell culture was not antagonistic when combined with the NNRTIs delavirdine, efavirenz, etravirine, nevirapine, or rilpivirine; the NRTIs abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir DF, or zidovudine; the PIs darunavir or indinavir; the gp41 fusion inhibitor enfuvirtide; the CCR5 co-receptor antagonist maraviroc; or the integrase strand transfer inhibitor raltegravir.
Resistance
In Cell Culture
Doravirine-resistant strains were selected in cell culture starting from wild-type HIV-1 of different origins and subtypes, as well as NNRTI-resistant HIV-1. Observed emergent amino acid substitutions in RT included: V106A, V106I, V106M, V108I, H221Y, F227C, F227I, F227L, F227V, M230I, L234I, P236L, and Y318F. The V106A, V106M, V108I, H221Y, F227C, M230I, P236L, and Y318F substitutions conferred 3.4-fold to 70-fold reductions in susceptibility to doravirine. Y318F in combination with V106A, V106M, V108I, or F227C conferred greater decreases in susceptibility to doravirine than Y318F alone, which conferred a 10-fold reduction in susceptibility to doravirine.
In Clinical Trials
Clinical Trial Results in Adults with No Antiretroviral Treatment History
In the doravirine treatment arms of the DRIVE-FORWARD and DRIVE-AHEAD trials (n=747) through Week 96, 13 participants showed the emergence of doravirine resistance-associated substitutions in their HIV among 36 (36%) participants in the resistance analysis subset (participants with HIV-1 RNA greater than 400 copies per mL at virologic failure or early study discontinuation and having post-baseline resistance samples). Emergent doravirine resistance-associated substitutions in RT included one or more of the following: V90G/I, A98G, V106A, V106I, V106M/T, V108I, E138G, Y188L, H221Y, P225H, P225L, P225P/S, F227C, F227C/R, Y318Y/F and Y318Y/S. Eight of 13 (62%) participants with emergent doravirine resistance-associated substitutions showed doravirine phenotypic resistance and most of them had at least a 100-fold reduction in doravirine susceptibility (range >95- to >211–fold reduction in doravirine susceptibility). The other 5 virologic failures who had only amino acid mixtures of NNRTI resistance substitutions showed doravirine phenotypic fold-changes of less than 2-fold. Of the 36 participants in the resistance analysis subset, 10 participants (28%) developed genotypic and/or phenotypic resistance to the other drugs (abacavir, emtricitabine, lamivudine, or tenofovir) in the regimens of the DRIVE-FORWARD and DRIVE-AHEAD trials. The resistance-associated substitutions that emerged were RT M41L (n=1), A62A/V (n=1), K65R (n=2), T69T/A (n=1), V75V/I (n=1), and M184I or V (n=7).
In the DRV/r treatment arm of the DRIVE-FORWARD trial (n=383) through Week 96, no participants showed the emergence of darunavir resistance-associated substitutions among 15 participants with resistance data and 2 of the participants had emergent genotypic or phenotypic resistance to lamivudine or tenofovir. In the EFV/FTC/TDF treatment arm of the DRIVE-AHEAD trial (n=364) through Week 96, 15 participants showed the emergence of efavirenz resistance-associated substitutions among 25 (60%) participants in the resistance analysis subset and genotypic resistance to emtricitabine or tenofovir developed in 5 evaluable participants; emergent resistance-associated substitutions were RT K65R (n=1), D67G/K70E (n=1), L74V/V75M/V118I (n=1), M184I or V (n=5), and K219K/E (n=1).
Clinical Trial Results in Virologically-Suppressed Adults
In the DRIVE-SHIFT clinical trial [see Clinical Studies (14.2) ] , there were 6 participants in the immediate switch group (n=447) and 2 participants in the delayed switch group (n=209) who met the protocol-defined virologic failure criteria (confirmed HIV-1 RNA ≥ 50 copies/mL). Two of the 6 virologic failure participants in the immediate switch group had available resistance data and neither developed detectable genotypic or phenotypic resistance to doravirine, lamivudine, or tenofovir during treatment with DELSTRIGO. One of the two virologic failure participants in the delayed switch group who had available resistance data developed the RT M184M/I substitution and phenotypic resistance to emtricitabine and lamivudine during treatment with their baseline regimen.
Cross-Resistance
Cross-resistance has been observed among NNRTIs. Treatment-emergent doravirine resistance-associated substitutions can confer cross-resistance to efavirenz, etravirine, nevirapine, and rilpivirine. Of the 8 virologic failure participants who developed doravirine phenotypic resistance, all had phenotypic resistance to nevirapine, 6 had phenotypic resistance to efavirenz, 4 had phenotypic resistance to rilpivirine, and 4 had resistance to etravirine in the Monogram PhenoSense assay. Of the 11 virologic failure participants in DRIVE-AHEAD phenotypically resistant to efavirenz, 2 (18%) had decreased susceptibility to doravirine (18- and 36-fold).
The treatment-emergent doravirine resistance-associated substitution Y318F did not confer reduced susceptibility to efavirenz, etravirine, or rilpivirine.
A panel of 96 diverse clinical isolates containing NNRTI resistance-associated substitutions was evaluated for susceptibility to doravirine. Clinical isolates containing the Y188L substitution alone or in combination with K103N or V106I, V106A in combination with G190A and F227L, or E138K in combination with Y181C and M230L showed greater than 100-fold reduced susceptibility to doravirine.
NONCLINICAL TOXICOLOGY
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Doravirine was not carcinogenic in long-term oral carcinogenicity studies in mice and rats at exposures up to 6 and 7 times, respectively, the human exposures at the RHD. A statistically significant incidence of thyroid parafollicular cell adenoma and carcinoma seen only in female rats at the high dose was within the range observed in historical controls.
Mutagenesis
Doravirine was not genotoxic in a battery of in vitro or in vivo assays, including microbial mutagenesis, chromosomal aberration in Chinese hamster ovary cells, and in in vivo rat micronucleus assays.
Impairment of fertility
There were no effects on fertility, mating performance or early embryonic development when doravirine was administered to rats at systemic exposures (AUC) approximately 7 times the exposure in humans at the RHD.
CLINICAL STUDIES
Clinical Trial Results in Adults with No Antiretroviral Treatment History
The efficacy of PIFELTRO is based on the analyses of 96-week data from two randomized, multicenter, double-blind, active controlled Phase 3 trials (DRIVE-FORWARD, NCT02275780 and DRIVE-AHEAD, NCT02403674) in participants living with HIV with no antiretroviral treatment history (n=1494).
In DRIVE-FORWARD, 766 participants were randomized and received at least 1 dose of either PIFELTRO once daily or darunavir 800 mg + ritonavir 100 mg (DRV+r) once daily each in combination with emtricitabine/tenofovir DF (FTC/TDF) or abacavir/lamivudine (ABC/3TC) selected by the investigator. At baseline, the median age of participants was 33 years, 16% were female, 27% were Non-White, 4% had hepatitis B and/or C virus co-infection, 10% had a history of AIDS, 20% had HIV-1 RNA greater than 100,000 copies/mL, 86% had CD4+ T-cell count greater than 200 cells/mm 3 , 13% received ABC/3TC, and 87% received FTC/TDF; these characteristics were similar between treatment groups.
In DRIVE-AHEAD, 728 participants were randomized and received at least 1 dose of either DELSTRIGO (DOR/3TC/TDF) or EFV 600 mg/FTC 200 mg/TDF 300 mg once daily. At baseline, the median age of participants was 31 years, 15% were female, 52% were Non-White, 3% had hepatitis B or C co-infection, 14% had a history of AIDS, 21% had HIV-1 RNA greater than 100,000 copies/mL, and 88% had CD4+ T-cell count greater than 200 cells/mm 3 ; these characteristics were similar between treatment groups.
Week 96 outcomes for DRIVE-FORWARD and DRIVE-AHEAD are provided in Table 10. Side-by-side tabulation is to simplify presentation; direct comparisons across trials should not be made due to differing trial designs.
In DRIVE-FORWARD, the mean CD4+ T-cell counts in the PIFELTRO and DRV+r groups increased from baseline by 224 and 207 cells/mm 3 , respectively.
In DRIVE-AHEAD, the mean CD4+ T-cell counts in the DELSTRIGO and EFV/FTC/TDF groups increased from baseline by 238 and 223 cells/mm 3 , respectively.
| Outcome | DRIVE-FORWARD | DRIVE-AHEAD | ||
|---|---|---|---|---|
| PIFELTRO + 2 NRTIs Once Daily | DRV+r + 2 NRTIs Once Daily | DELSTRIGO Once Daily | EFV/FTC/TDF Once Daily | |
| N=383 | N=383 | N=364 | N=364 | |
| Note: NRTIs = FTC/TDF or ABC/3TC. | ||||
| HIV-1 RNA <50 copies/mL | 72% | 65% | 77% | 74% |
| Treatment Differences (95% CI) The 95% CIs for the treatment differences were calculated using stratum-adjusted Mantel-Haenszel method. | 7.5% (1.0%, 14.1%) | 3.8% (-2.4%, 10.0%) | ||
| HIV-1 RNA ≥ 50 copies/mL Includes participants who discontinued study drug or study before Week 96 for lack or loss of efficacy and participants with HIV-1 RNA equal to or above 50 copies/mL in the Week 96 window. | 17% | 20% | 15% | 12% |
| No Virologic Data at Week 96 Window | 11% | 15% | 7% | 14% |
| Discontinued study due to AE or Death Includes participants who discontinued because of adverse event (AE) or death if this resulted in no virologic data in the Week 96 window. | 2% | 4% | 3% | 8% |
| Discontinued study for Other Reasons Other Reasons include: lost to follow-up, non-compliance with study drug, physician decision, pregnancy, protocol deviation, screen failure, withdrawal by participant. | 7% | 9% | 4% | 5% |
| On study but missing data in window | 2% | 3% | 1% | 1% |
| Proportion (%) of Participants With HIV-1 RNA <50 copies/mL at Week 96 by Baseline and Demographic Category | ||||
| Gender | ||||
| Male | 72% (N = 319) | 67% (N = 326) | 78% (N = 305) | 73% (N = 311) |
| Female | 73% (N = 64) | 54% (N = 57) | 75% (N = 59) | 75% (N = 53) |
| Race | ||||
| White | 78% (N = 280) | 68% (N = 280) | 80% (N = 176) | 74% (N = 170) |
| Non-White | 58% (N = 103) | 57% (N = 102) | 76% (N = 188) | 74% (N = 194) |
| Ethnicity Does not include participants whose ethnicity or viral subtypes were unknown. | ||||
| Hispanic or Latino | 76% (N = 93) | 63% (N = 86) | 81% (N = 126) | 77% (N = 119) |
| Not Hispanic or Latino | 71% (N = 284) | 66% (N = 290) | 76% (N = 238) | 72% (N = 239) |
| NRTI Background Therapy | ||||
| FTC/TDF | 71% (N = 333) | 64% (N = 335) | - | - |
| ABC/3TC | 80% (N = 50) | 67% (N = 48) | - | - |
| Baseline HIV-1 RNA (copies/mL) | ||||
| ≤100,000 copies/mL | 75% (N = 300) | 66% (N = 309) | 80% (N = 291) | 77% (N = 282) |
| >100,000 copies/mL | 61% (N = 83) | 59% (N = 73) | 67% (N = 73) | 62% (N = 82) |
| CD4+ T-cell Count (cells/mm 3 ) | ||||
| ≤200 cells/mm 3 | 62% (N = 42) | 51% (N = 67) | 59% (N = 44) | 70% (N = 46) |
| >200 cells/mm 3 | 74% (N = 341) | 68% (N = 316) | 80% (N = 320) | 74% (N = 318) |
| Viral Subtype | ||||
| Subtype B | 71% (N = 266) | 66% (N = 272) | 80% (N = 232) | 72% (N = 253) |
| Subtype Non-B | 75% (N = 117) | 62% (N = 111) | 73% (N = 130) | 77% (N = 111) |
Clinical Trial Results in Virologically-Suppressed Adults
The efficacy of switching from a baseline regimen consisting of two NRTIs in combination with a PI plus either ritonavir or cobicistat, or elvitegravir plus cobicistat, or an NNRTI to DELSTRIGO was evaluated in a randomized, open-label trial (DRIVE-SHIFT, NCT02397096), in virologically-suppressed adults living with HIV. Participants must have been virologically-suppressed (HIV-1 RNA < 50 copies/mL) on their baseline regimen for at least 6 months prior to trial entry, with no history of virologic failure. Participants were randomized to either switch to DELSTRIGO at baseline (n = 447, Immediate Switch Group (ISG)), or stay on their baseline regimen until Week 24, at which point they switched to DELSTRIGO (n = 223, Delayed Switch Group (DSG)).
At baseline, the median age of participants was 43 years, 16% were female, and 24% were Non-White, 21% were of Hispanic or Latino ethnicity, 3% had hepatitis B and/or C virus co-infection, 17% had a history of AIDS, 96% had CD4+ T-cell count greater than or equal to 200 cells/mm 3 , 70% were on a regimen containing a PI plus ritonavir, 24% were on a regimen containing an NNRTI, 6% were on a regimen containing elvitegravir plus cobicistat, and 1% were on a regimen containing a PI plus cobicistat; these characteristics were similar between treatment groups.
Virologic outcome results are shown in Table 11.
| Outcome | DELSTRIGO Once Daily ISG Week 48 N=447 | Baseline Regimen DSG Week 24 N=223 |
|---|---|---|
| HIV-1 RNA ≥ 50 copies/mL Includes participants who discontinued study drug or study before Week 48 for ISG or before Week 24 for DSG for lack or loss of efficacy and participants with HIV-1 RNA ≥50 copies/mL in the Week 48 window for ISG and in the Week 24 window for DSG. | 2% | 1% |
| ISG-DSG, Difference (95% CI) The 95% CI for the treatment difference was calculated using stratum-adjusted Mantel-Haenszel method. , Assessed using a non-inferiority margin of 4%. | 0.7% (-1.3%, 2.6%) | |
| HIV-1 RNA <50 copies/mL | 91% | 95% |
| No Virologic Data Within the Time Window | 8% | 4% |
| Discontinued study due to AE or Death Includes participants who discontinued because of adverse event (AE) or death if this resulted in no virologic data on treatment during the specified window. | 3% | <1% |
| Discontinued study for Other Reasons Other reasons include: lost to follow-up, non-compliance with study drug, physician decision, protocol deviation, withdrawal by participant. | 4% | 4% |
| On study but missing data in window | 0 | 0 |
| Proportion (%) of Participants With HIV-1 RNA <50 copies/mL by Baseline and Demographic Category | ||
| Age (years) | ||
| < 50 | 90% (N = 320) | 95% (N = 157) |
| ≥ 50 | 94% (N = 127) | 94% (N = 66) |
| Gender | ||
| Male | 91% (N = 372) | 94% (N = 194) |
| Female | 91% (N = 75) | 100% (N = 29) |
| Race | ||
| White | 90% (N = 344) | 95% (N = 168) |
| Non-White | 93% (N = 103) | 93% (N = 55) |
| Ethnicity | ||
| Hispanic or Latino | 88% (N = 99) | 91% (N = 45) |
| Not Hispanic or Latino | 91% (N = 341) | 95% (N = 175) |
| CD4+ T-cell Count (cells/mm 3 ) | ||
| <200 cells/mm 3 | 85% (N = 13) | 75% (N = 4) |
| ≥200 cells/mm 3 | 91% (N = 426) | 95% (N = 216) |
| Baseline Regimen Baseline Regimen = PI plus either ritonavir or cobicistat (specifically atazanavir, darunavir, or lopinavir), or elvitegravir plus cobicistat, or NNRTI (specifically efavirenz, nevirapine, or rilpivirine), each administered with two NRTIs. | ||
| PI plus either ritonavir or cobicistat | 90% (N=316) | 94% (N=156) |
| elvitegravir plus cobicistat or NNRTI | 93% (N=131) | 96% (N=67) |
Clinical Trial Results in Pediatric Participants
The efficacy of DELSTRIGO (DOR/3TC/TDF) was evaluated in cohort 2 of an open-label, single-arm 2-cohort trial in pediatric participants 12 to less than 18 years of age living with HIV (IMPAACT 2014 (Protocol 027), NCT03332095). In cohort 1, virologically-suppressed participants (n=9) received a single 100 mg dose of PIFELTRO followed by intensive PK sampling. In cohort 2, virologically-suppressed participants (n=43) were switched to DELSTRIGO and treatment-naïve participants (n=2) were started on DELSTRIGO.
In cohort 2, at baseline the median age of participants was 15 years (range: 12 to 17), the median weight was 52 kg (range: 45 to 80), 58% were female, 78% were Asian and 22% were Black, and the median CD4+ T-cell count was 713 cells per mm 3 (range 84 to 1397). After switching to DELSTRIGO, 95% (41/43) of virologically-suppressed participants remained suppressed (HIV-1 RNA <50 copies/mL) at Week 24. One of the two treatment-naïve participants achieved HIV-1 RNA <50 copies/mL at Week 24. The other treatment-naïve participant met the protocol-defined virologic failure criteria (defined as 2 consecutive plasma HIV-1 RNA test results ≥200 copies/mL at or after Week 24) and was evaluated for the development of resistance; no emergence of genotypic or phenotypic resistance to doravirine, lamivudine, or tenofovir was detected.
HOW SUPPLIED/STORAGE AND HANDLING
Each PIFELTRO tablet contains 100 mg of doravirine, is white, oval-shaped and film-coated, and is debossed with the corporate logo and 700 on one side and plain on the other side. Each bottle contains 30 tablets (NDC 0006-3069-01) with silica gel desiccant and is closed with a child-resistant closure.
Store PIFELTRO in the original bottle. Keep the bottle tightly closed to protect from moisture. Do not remove the desiccant.
Store PIFELTRO at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Mechanism of Action
Doravirine is an antiretroviral drug [see Microbiology (12.4) ].